Herpes simplex virus (HSV)-specific T cells activated in the absence of IFN-gamma express alternative effector functions but are not protective against genital HSV-2 infection
- PMID: 19942296
- PMCID: PMC2815080
- DOI: 10.1016/j.jri.2009.09.007
Herpes simplex virus (HSV)-specific T cells activated in the absence of IFN-gamma express alternative effector functions but are not protective against genital HSV-2 infection
Abstract
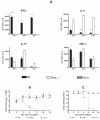
Interferon gamma (IFNgamma) is important for immune resistance to herpes simplex virus (HSV) infection. To examine the influence of IFNgamma on the development of HSV-specific immune responses and test for IFNgamma-independent adaptive immune mechanisms of protection, IFNgamma-deficient mice (IFNgamma(-/-)) were immunized with thymidine kinase-deficient HSV-2 (HSV-2 333tk(-)). HSV-specific cellular and humoral responses were elicited in immunized IFNgamma(-/-) mice resulting in increased resistance relative to non-immune C57BL/6J (B6) mice following challenge with fully virulent HSV-2. CD8(+) T cells from IFNgamma(-/-) mice displayed cytotoxic activity and secreted TNFalpha. HSV-specific CD4(+) T cells from immunized IFNgamma(-/-) mice secreted IL-4, TNFalpha, and IL-17, but unlike T cells from HSV-immune B6 mice, could not clear virus from genital tissue following adoptive transfer. HSV-immune IFNgamma(-/-) mice produced predominantly IgG(1) HSV-specific antibodies while immune B6 mice produced predominantly IgG(2c) antibodies. Transfer of equivalent amounts of HSV-specific antibodies from either strain to naïve mice imparted equivalent early resistance against infection of the genital epithelia. However, protection against neurological symptoms mediated by immune B6 antibodies was superior late in infection. Taken together, these results demonstrate that the limited resistance of HSV-immune IFNgamma(-/-) mice to HSV-2 infection resulted from the action of HSV-specific Ab rather than IFNgamma-independent effector functions of T cells. Further, protection against neurological manifestations of HSV-2 infection was superior in mice receiving Ab from immune B6 mice suggesting that Ab-mediated protective mechanisms involving IFNgamma-induced IgG subclasses were more effective once virus had spread to neural tissues.
2009 Elsevier Ireland Ltd. All rights reserved.
Figures
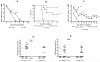
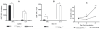

Similar articles
-
Rapid clearance of herpes simplex virus type 2 by CD8+ T cells requires high level expression of effector T cell functions.J Reprod Immunol. 2011 Apr;89(1):10-7. doi: 10.1016/j.jri.2011.01.013. Epub 2011 Mar 27. J Reprod Immunol. 2011. PMID: 21444117 Free PMC article.
-
Differential roles of B cells and IFN-gamma-secreting CD4(+) T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice.J Gen Virol. 2001 Apr;82(Pt 4):845-853. doi: 10.1099/0022-1317-82-4-845. J Gen Virol. 2001. PMID: 11257190
-
Novel Role for Interleukin-17 in Enhancing Type 1 Helper T Cell Immunity in the Female Genital Tract following Mucosal Herpes Simplex Virus 2 Vaccination.J Virol. 2017 Nov 14;91(23):e01234-17. doi: 10.1128/JVI.01234-17. Print 2017 Dec 1. J Virol. 2017. PMID: 28956763 Free PMC article.
-
Overexpression of interleukin-15 compromises CD4-dependent adaptive immune responses against herpes simplex virus 2.J Virol. 2009 Jan;83(2):918-26. doi: 10.1128/JVI.01282-08. Epub 2008 Nov 12. J Virol. 2009. PMID: 19004955 Free PMC article.
-
Therapeutic Mucosal Vaccination of Herpes Simplex Virus 2-Infected Guinea Pigs with Ribonucleotide Reductase 2 (RR2) Protein Boosts Antiviral Neutralizing Antibodies and Local Tissue-Resident CD4+ and CD8+ TRM Cells Associated with Protection against Recurrent Genital Herpes.J Virol. 2019 Apr 17;93(9):e02309-18. doi: 10.1128/JVI.02309-18. Print 2019 May 1. J Virol. 2019. PMID: 30787156 Free PMC article.
Cited by
-
Mature dendritic cells cause Th17/Treg imbalance by secreting TGF-β1 and IL-6 in the pathogenesis of experimental autoimmune encephalomyelitis.Cent Eur J Immunol. 2016;41(2):143-52. doi: 10.5114/ceji.2016.60987. Epub 2016 Jul 15. Cent Eur J Immunol. 2016. PMID: 27536199 Free PMC article.
-
Herpes Simplex Virus Evasion of Early Host Antiviral Responses.Front Cell Infect Microbiol. 2019 Apr 30;9:127. doi: 10.3389/fcimb.2019.00127. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31114761 Free PMC article. Review.
-
Immunoregulatory Functions of Interferons During Genital HSV-2 Infection.Front Immunol. 2021 Aug 18;12:724618. doi: 10.3389/fimmu.2021.724618. eCollection 2021. Front Immunol. 2021. PMID: 34484233 Free PMC article. Review.
-
Rapid clearance of herpes simplex virus type 2 by CD8+ T cells requires high level expression of effector T cell functions.J Reprod Immunol. 2011 Apr;89(1):10-7. doi: 10.1016/j.jri.2011.01.013. Epub 2011 Mar 27. J Reprod Immunol. 2011. PMID: 21444117 Free PMC article.
-
Immunogenicity of RepliVAX WN, a novel single-cycle West Nile virus vaccine.Vaccine. 2010 Dec 16;29(2):174-82. doi: 10.1016/j.vaccine.2010.10.069. Epub 2010 Nov 4. Vaccine. 2010. PMID: 21055493 Free PMC article.
References
-
- Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2001;290:1354–1358. - PubMed
-
- Bouley DM, Kanangat S, Wire W, Rouse BT. Characterization of herpes simplex virus type -1 infection and herpetic stromal keratitis development in IFNγ knockout mice. J. Immunol. 1995;155:3964–3971. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

