CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes
- PMID: 19942623
- PMCID: PMC2819835
- DOI: 10.1093/cvr/cvp369
CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes
Abstract
Aims: Here we investigated the mechanisms by which cardiovascular CB1 cannabinoid receptors may modulate the cardiac dysfunction, oxidative stress, and interrelated cell death pathways associated with acute/chronic cardiomyopathy induced by the widely used anti-tumour compound doxorubicin (DOX).
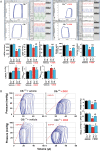
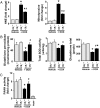
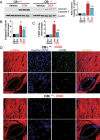
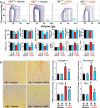
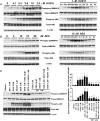
Methods and results: Both load-dependent and -independent indices of left-ventricular function were measured by the Millar pressure-volume conductance system. Mitogen-activated protein kinase (MAPK) activation, cell-death markers, and oxidative/nitrosative stress were measured by molecular biology/biochemical methods and flow cytometry. DOX induced left-ventricular dysfunction, oxidative/nitrosative stress coupled with impaired antioxidant defense, activation of MAPK (p38 and JNK), and cell death and/or fibrosis in hearts of wide-type mice (CB1(+/+)), and these effects were markedly attenuated in CB1 knockouts (CB1(-/-)). In human primary cardiomyocytes expressing CB1 receptors (demonstrated by RT-PCR, western immunoblot, and flow cytometry) DOX, likewise the CB1 receptor agonist HU210 and the endocannabinoid anandamide (AEA), induced MAPK activation and cell death. The DOX-induced MAPK activation and cell death were significantly enhanced when DOX was co-administered with CB1 agonists AEA or HU210. Remarkably, cell death and MAPK activation induced by AEA, HU210, and DOX +/- AEA/HU210 were largely attenuated by either CB1 antagonists (rimonabant and AM281) or by inhibitors of p38 and JNK MAPKs. Furthermore, AEA or HU210 in primary human cardiomyocytes triggered increased reactive oxygen species generation.
Conclusion: CB1 activation in cardiomyocytes may amplify the reactive oxygen/nitrogen species-MAPK activation-cell death pathway in pathological conditions when the endocannabinoid synthetic or metabolic pathways are dysregulated by excessive inflammation and/or oxidative/nitrosative stress, which may contribute to the pathophysiology of various cardiovascular diseases.
Figures

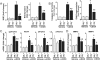
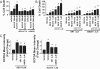
References
-
- Engeli S. Dysregulation of the endocannabinoid system in obesity. J Neuroendocrinol. 2008;20(Suppl. 1):110–115. - PubMed
-
- Di Marzo V. The endocannabinoid system in obesity and type 2 diabetes. Diabetologia. 2008;51:1356–1367. - PubMed
-
- Sugamura K, Sugiyama S, Nozaki T, Matsuzawa Y, Izumiya Y, Miyata K, et al. Activated endocannabinoid system in coronary artery disease and antiinflammatory effects of cannabinoid 1 receptor blockade on macrophages. Circulation. 2009;119:28–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

