Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro
- PMID: 19943704
- PMCID: PMC2807922
- DOI: 10.1021/bi9015555
Disulfide-dependent self-assembly of adiponectin octadecamers from trimers and presence of stable octadecameric adiponectin lacking disulfide bonds in vitro
Abstract
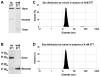
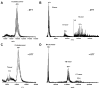
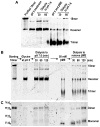
Adiponectin is a circulating insulin-sensitizing hormone that homooligomerizes into trimers, hexamers, and higher molecular weight (HMW) species. Low levels of circulating HMW adiponectin appear to increase the risk for insulin resistance. Currently, assembly of adiponectin oligomers and, consequently, mechanisms responsible for decreased HMW adiponectin in insulin resistance are not well understood. In the work reported here, we analyzed the reassembly of the most abundant HMW adiponectin species, the octadecamer, following its collapse to smaller oligomers in vitro. Purified bovine serum adiponectin octadecamer was treated with reducing agents at pH 5 to obtain trimers. These reduced trimers partially and spontaneously reassembled into octadecamers upon oxidative formation of disulfide bonds. Disulfide bonds appear to occupy a greater role in the process of oligomerization than in the structural stabilization of mature octadecamer. Stable octadecamers lacking virtually all disulfide bonds could be observed in abundance using native gel electrophoresis, dynamic light scattering, and collision-induced dissociation nanoelectrospray ionization mass spectrometry. These findings indicate that while disulfide bonds help to maintain the mature octadecameric adiponectin structure, their more important function is to stabilize intermediates during the assembly of octadecamer. Adiponectin oligomerization must proceed through intermediates that are at least partially reduced. Accordingly, fully oxidized adiponectin hexamers failed to reassemble into octadecamers at a rate comparable to that of reduced trimers. As the findings from the present study are based on in vitro experiments, their in vivo relevance remains unclear. Nevertheless, they describe a redox environment-dependent model of adiponectin oligomerization that can be tested using cell-based approaches.
Figures



References
-
- Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32(Suppl 7):S13–18. - PubMed
-
- Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. - PubMed
-
- Tsao TS, Lodish HF, Fruebis J. ACRP30, a new hormone controlling fat and glucose metabolism. Eur J Pharmacol. 2002;440:213–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

