Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans
- PMID: 19943902
- PMCID: PMC3699866
- DOI: 10.1111/j.1365-2958.2009.06983.x
Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans
Abstract
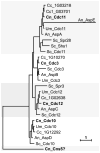
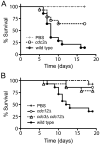
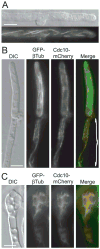
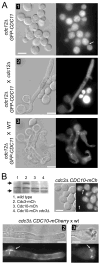
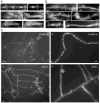
Septins are conserved, cytoskeletal GTPases that contribute to cytokinesis, exocytosis, cell surface organization and vesicle fusion by mechanisms that are poorly understood. Roles of septins in morphogenesis and virulence of a human pathogen and basidiomycetous yeast Cryptococcus neoformans were investigated. In contrast to a well-established paradigm in S. cerevisiae, Cdc3 and Cdc12 septin homologues are dispensable for growth in C. neoformans yeast cells at 24 degrees C but are essential at 37 degrees C. In a bilateral cross between septin mutants, cells fuse but the resulting hyphae exhibit morphological abnormalities, including lack of properly fused specialized clamp cells and failure to produce spores. Interestingly, post-mating hyphae of the septin mutants have a defect in nuclear distribution. Thus, septins are essential for the development of spores, clamp cell fusion and also play a specific role in nuclear dynamics in hyphae. In the post-mating hyphae the septins localize to discrete sites in clamp connections, to the septa and the bases of the initial emerging spores. Strains lacking CDC3 or CDC12 exhibit significantly reduced virulence in a Galleria mellonella model of infection. Thus, C. neoformans septins are vital to morphology of the hyphae and contribute to virulence.
Figures



References
-
- Badalyan SM, Polak E, Hermann R, Aebi M, Kues U. Role of peg formation in clamp cell fusion of homobasidiomycete fungi. J Basic Microbiol. 2004;44:167–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

