Desmozoon lepeophtherii n. gen., n. sp., (Microsporidia: Enterocytozoonidae) infecting the salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae)
- PMID: 19943930
- PMCID: PMC2791097
- DOI: 10.1186/1756-3305-2-58
Desmozoon lepeophtherii n. gen., n. sp., (Microsporidia: Enterocytozoonidae) infecting the salmon louse Lepeophtheirus salmonis (Copepoda: Caligidae)
Abstract
Background: A microsporidian was previously reported to infect the crustacean parasite, Lepeophtheirus salmonis (Krøyer, 1837) (Copepoda, Caligidae), on farmed Atlantic salmon (Salmo salar L.) in Scotland. The microsporidian was shown to be a novel species with a molecular phylogenetic relationship to Nucleospora (Enterocytozoonidae), but the original report did not assign it to a genus or species. Further studies examined the development of the microsporidian in L. salmonis using electron microscopy and re-evaluated the molecular findings using new sequence data available for the group. Here we report a full description for the microsporidian and assign it to a new genus and species.
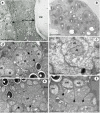
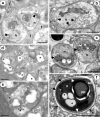
Results: The microsporidian infects subcuticular cells that lie on the innermost region of the epidermal tissue layer beneath the cuticle and along the internal haemocoelic divisions. The mature spores are sub-spherical with a single nucleus and an isofilar polar filament with 5-8 turns in a double coil. The entire development is in direct contact with the host cell cytoplasm and is polysporous. During early merogony, a diplokaryotic nuclear arrangement exists which is absent throughout the rest of the developmental cycle. Large merogonial plasmodia form which divide to form single uninucleate sporonts. Sporogonial plasmodia were not observed; instead, binucleate sporonts divide to form two sporoblasts. Prior to final division, there is a precocious development of the polar filament extrusion apparatus which is associated with large electron lucent inclusions (ELIs). Analyses of DNA sequences reveal that the microsporidian is robustly supported in a clade with other members of the Enterocytozoonidae and confirms a close phylogenetic relationship with Nucleospora.
Conclusion: The ultrastructural findings of the precocious development of the polar filament and the presence of ELIs are consistent with those of the Enterocytozoonidae. However, the confirmed presence of an early diplokaryotic stage and a merogonial plasmodium that divides to yield uninucleate sporonts instead of transforming into a sporogonial syncitium, are features not currently associated with the family. Yet, analyses of DNA sequence data clearly place the microsporidian within the Enterocytozoonidae. Therefore, due to the novelty of the copepod host, the ultrastructural findings and the robust nature of the phylogenetic analyses, a new genus should be created within the Enterocytozoonide; Desmozoon lepeophtherii n. gen. n. sp. is proposed.
Figures




Similar articles
-
Paranucleospora theridion n. gen., n. sp. (Microsporidia, Enterocytozoonidae) with a Life Cycle in the Salmon Louse (Lepeophtheirus salmonis, Copepoda) and Atlantic Salmon (Salmo salar).J Eukaryot Microbiol. 2010 Mar-Apr;57(2):95-114. doi: 10.1111/j.1550-7408.2009.00451.x. Epub 2010 Jan 11. J Eukaryot Microbiol. 2010. PMID: 20070452
-
Original observations of Desmozoon lepeophtherii, a microsporidian hyperparasite infecting the salmon louse Lepeophtheirus salmonis, and its subsequent detection by other researchers.Parasit Vectors. 2011 Dec 13;4:231. doi: 10.1186/1756-3305-4-231. Parasit Vectors. 2011. PMID: 22166354 Free PMC article.
-
A new intranuclear microsporidium, Enterospora nucleophila n. sp., causing an emaciative syndrome in a piscine host (Sparus aurata), prompts the redescription of the family Enterocytozoonidae.Int J Parasitol. 2014 Mar;44(3-4):189-203. doi: 10.1016/j.ijpara.2013.10.005. Epub 2013 Dec 8. Int J Parasitol. 2014. PMID: 24326177
-
Pseudokabatana alburnus n. gen. n. sp., (Microsporidia) from the liver of topmouth culter Culter alburnus (Actinopterygii, Cyprinidae) from China.Parasitol Res. 2019 Jun;118(6):1689-1699. doi: 10.1007/s00436-019-06303-z. Epub 2019 Apr 11. Parasitol Res. 2019. PMID: 30976967 Review.
-
Fish microsporidia: fine structural diversity and phylogeny.Int J Parasitol. 2003 Feb;33(2):107-27. doi: 10.1016/s0020-7519(02)00252-7. Int J Parasitol. 2003. PMID: 12633649 Review.
Cited by
-
Intranuclear inclusions consistent with a Nucleospora sp. in a lymphoid lesion in a laboratory zebrafish, Danio rerio (Hamilton 1822).J Fish Dis. 2021 Jan;44(1):107-112. doi: 10.1111/jfd.13271. Epub 2020 Oct 24. J Fish Dis. 2021. PMID: 33098687 Free PMC article. No abstract available.
-
Nucleospora hippocampi n. sp., an Intranuclear Microsporidian Infecting the Seahorse Hippocampus erectus From China.Front Cell Infect Microbiol. 2022 May 4;12:882843. doi: 10.3389/fcimb.2022.882843. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35601100 Free PMC article.
-
Nucleospora cyclopteri n. sp., an intranuclear microsporidian infecting wild lumpfish, Cyclopterus lumpus L., in Icelandic waters.Parasit Vectors. 2013 Feb 27;6:49. doi: 10.1186/1756-3305-6-49. Parasit Vectors. 2013. PMID: 23445616 Free PMC article.
-
Development and characterization of two cell lines from gills of Atlantic salmon.PLoS One. 2018 Feb 14;13(2):e0191792. doi: 10.1371/journal.pone.0191792. eCollection 2018. PLoS One. 2018. PMID: 29444101 Free PMC article.
-
Effects of cnidarian biofouling on salmon gill health and development of amoebic gill disease.PLoS One. 2018 Jul 6;13(7):e0199842. doi: 10.1371/journal.pone.0199842. eCollection 2018. PLoS One. 2018. PMID: 29979703 Free PMC article.
References
-
- Sprague V, Couch J. An annotated list of protozoan parasites, hyperparasites and commensals of decapod crustacea. J Protozool. 1971;18:526–537.
-
- Olson RE, Tiekotter KL, Reno PW. Nadelspora canceri n.g., n.s. an unusual microsporidian parasite of the Dungeness crab, Cancer magister. J Euk Microbiol. 1994;41:349–359. doi: 10.1111/j.1550-7408.1994.tb06089.x. - DOI
-
- Martinez MA, Vivares CP, Rocha RD, Fonseca AC, Andral B, Bouix G. Microsporidiosis on Artemia (Crustacea, Anostraca) Light and electron microscopy of Vavraia anostraca sp. nov. (Microsporidia: Pleistophoridae) in the Brazilian Solar Salterns. Aquaculture. 1992;107:229–237. doi: 10.1016/0044-8486(92)90071-R. - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

