Smoking cessation or reduction with nicotine replacement therapy: a placebo-controlled double blind trial with nicotine gum and inhaler
- PMID: 19943947
- PMCID: PMC2792228
- DOI: 10.1186/1471-2458-9-433
Smoking cessation or reduction with nicotine replacement therapy: a placebo-controlled double blind trial with nicotine gum and inhaler
Abstract
Background: Even with effective smoking cessation medications, many smokers are unable to abruptly stop using tobacco. This finding has increased interest in smoking reduction as an interim step towards complete cessation.
Methods: This multi-center, double-blind placebo-controlled study evaluated the efficacy and safety of nicotine 4 mg gum or nicotine 10 mg inhaler in helping smokers (N = 314) to reduce or quit smoking. It included smokers willing to control their smoking, and participants could set individual goals, to reduce or quit. The study was placebo-controlled, randomized in a ratio of 2:1 (Active:Placebo), and subjects could choose inhaler or gum after randomization. Outcome was short-term (from Week 6 to Month 4) and long-term (from Month 6 to Month 12) abstinence or reduction. Abstinence was defined as not a single cigarette smoked and expired CO readings of <10 ppm. Smoking reduction was defined as a reduction in number of cigarettes per day by 50% or more versus baseline, verified by a lower-than-baseline CO reading at each visit during the same periods.
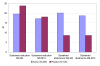
Results: Significantly more smokers managed to quit in the Active group than in the Placebo group. Sustained abstinence rates at 4 months were 42/209 (20.1%) subjects in the Active group and 9/105 (8.6%) subjects in the Placebo group (p = 0.009). Sustained abstinence rates at 12 months were 39/209 (18.7%) and 9/105 (8.6%), respectively (p = 0.019). Smoking reduction did not differ between the groups, either at short-term or long-term. Twelve-month reduction results were 17.2% vs. 18.1%, respectively. No serious adverse events were reported.
Conclusion: In conclusion, treatment with 10 mg nicotine inhaler or 4 mg nicotine chewing gum resulted in a significantly higher abstinence rate than placebo. In addition a large number of smokers managed to reduce their cigarette consumption by more than 50% compared to baseline.
Figures


References
-
- Stead LF, Perera R, Bullen C. Nicotine replacement therapy for smoking cessation (review) Cochrane Database of Systematic Reviews. 2008. p. CD000146. DOI: 10.1002/14651858.CD000146.pub3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

