Critical involvement of Rho GTPase activity in the efficient transplantation of neural stem cells into the injured spinal cord
- PMID: 19943951
- PMCID: PMC2789715
- DOI: 10.1186/1756-6606-2-37
Critical involvement of Rho GTPase activity in the efficient transplantation of neural stem cells into the injured spinal cord
Abstract
Background: Transplantation of neural stem/progenitor cells is a promising approach toward functional restoration of the damaged neural tissue, but the injured spinal cord has been shown to be an adverse environment for the survival, migration, and differentiation of the donor cells. To improve the efficiency of cell replacement therapy, cell autonomous factors in the donor cells should be optimized. In light of recent findings that Rho family GTPases regulate stem cell functions, genetic manipulation of Rho GTPases can potentially control phenotypes of transplanted cells. Therefore we expressed mutant forms of Rho GTPases, Rac, Rho, and Cdc42, in the neural stem/progenitor cells and examined their survival and migration after transplantation.
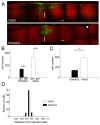
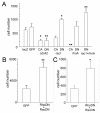
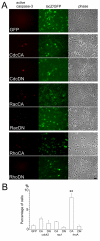
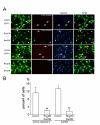
Results: Manipulation of the individual Rho GTPases showed differential effects on survival, with little variation in their migratory route and predominant differentiation into the oligodendroglial lineage. Combined suppression of both Rac and Rho activity had a prominent effect on promoting survival, consistent with its highly protective effect on drug-induced apoptosis in culture.
Conclusion: Manipulation of Rac and Rho activities fully rescued suppression of cell survival induced by the spinal cord injury. Our results indicate that precise regulation of cell autonomous factors within the donor cells can ameliorate the detrimental environment created by the injury.
Figures



Similar articles
-
Combination of edaravone and neural stem cell transplantation repairs injured spinal cord in rats.Genet Mol Res. 2015 Dec 29;14(4):19136-43. doi: 10.4238/2015.December.29.23. Genet Mol Res. 2015. PMID: 26782566
-
Effects of dibutyryl cyclic-AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats.PLoS One. 2011;6(6):e21744. doi: 10.1371/journal.pone.0021744. Epub 2011 Jun 30. PLoS One. 2011. PMID: 21738784 Free PMC article.
-
Targeted Inhibition of Leucine-Rich Repeat and Immunoglobulin Domain-Containing Protein 1 in Transplanted Neural Stem Cells Promotes Neuronal Differentiation and Functional Recovery in Rats Subjected to Spinal Cord Injury.Crit Care Med. 2016 Mar;44(3):e146-57. doi: 10.1097/CCM.0000000000001351. Crit Care Med. 2016. PMID: 26491860
-
Mevalonate Cascade and Small Rho GTPase in Spinal Cord Injury.Curr Mol Pharmacol. 2017;10(2):141-151. doi: 10.2174/1874467209666160112123322. Curr Mol Pharmacol. 2017. PMID: 26758952 Review.
-
The role of Rho GTPases' substrates Rac and Cdc42 in osteoclastogenesis and relevant natural medicinal products study.Biosci Rep. 2020 Jul 31;40(7):BSR20200407. doi: 10.1042/BSR20200407. Biosci Rep. 2020. PMID: 32578854 Free PMC article. Review.
Cited by
-
Rho protein GTPases and their interactions with NFκB: crossroads of inflammation and matrix biology.Biosci Rep. 2014 Jun 25;34(3):e00115. doi: 10.1042/BSR20140021. Biosci Rep. 2014. PMID: 24877606 Free PMC article. Review.
-
Lineage tracing reveals transient phenotypic adaptation of tubular cells during acute kidney injury.iScience. 2024 Feb 16;27(3):109255. doi: 10.1016/j.isci.2024.109255. eCollection 2024 Mar 15. iScience. 2024. PMID: 38444605 Free PMC article.
-
Identification of crucial genes associated with rat traumatic spinal cord injury.Mol Med Rep. 2017 Apr;15(4):1997-2006. doi: 10.3892/mmr.2017.6267. Epub 2017 Mar 1. Mol Med Rep. 2017. PMID: 28260098 Free PMC article.
-
Cell Therapy From Bench to Bedside Translation in CNS Neurorestoratology Era.Cell Med. 2010 Jan 1;1(1):15-46. doi: 10.3727/215517910X516673. Cell Med. 2010. PMID: 21359168 Free PMC article.
-
Crosstalk between the Rho and Rab family of small GTPases in neurodegenerative disorders.Front Cell Neurosci. 2023 Jan 27;17:1084769. doi: 10.3389/fncel.2023.1084769. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36779014 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

