Generalized immune activation as a direct result of activated CD4+ T cell killing
- PMID: 19943952
- PMCID: PMC2790834
- DOI: 10.1186/jbiol194
Generalized immune activation as a direct result of activated CD4+ T cell killing
Abstract
Background: In addition to progressive CD4(+) T cell immune deficiency, HIV infection is characterized by generalized immune activation, thought to arise from increased microbial exposure resulting from diminishing immunity.
Results: Here we report that, in a virus-free mouse model, conditional ablation of activated CD4(+) T cells, the targets of immunodeficiency viruses, accelerates their turnover and produces CD4(+) T cell immune deficiency. More importantly, activated CD4(+) T cell killing also results in generalized immune activation, which is attributable to regulatory CD4(+) T cell insufficiency and preventable by regulatory CD4(+) T cell reconstitution. Immune activation in this model develops independently of microbial exposure. Furthermore, microbial translocation in mice with conditional disruption of intestinal epithelial integrity affects myeloid but not T cell homeostasis.
Conclusions: Although neither ablation of activated CD4(+) T cells nor disruption of intestinal epithelial integrity in mice fully reproduces every aspect of HIV-associated immune dysfunction in humans, ablation of activated CD4(+) T cells, but not disruption of intestinal epithelial integrity, approximates the two key immune alterations in HIV infection: CD4(+) T cell immune deficiency and generalized immune activation. We therefore propose activated CD4(+) T cell killing as a common etiology for both immune deficiency and activation in HIV infection.
Figures

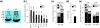


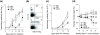


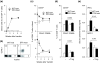
Comment in
-
Life and death as a T lymphocyte: from immune protection to HIV pathogenesis.J Biol. 2009;8(10):91. doi: 10.1186/jbiol198. J Biol. 2009. PMID: 19951397 Free PMC article. Review.
References
-
- Giorgi J, Hultin L, McKeating J, Johnson T, Owens B, Jacobson L, Shih R, Lewis J, Wiley D, Phair J, Wolinsky SM, Detels R. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. - DOI - PubMed
-
- Deeks SG, Kitchen CMR, Liu L, Guo H, Gascon R, Narvaez AB, Hunt P, Martin JN, Kahn JO, Levy J, McGrath MS, Hecht FM. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

