Go contributes to olfactory reception in Drosophila melanogaster
- PMID: 19943954
- PMCID: PMC2789035
- DOI: 10.1186/1472-6793-9-22
Go contributes to olfactory reception in Drosophila melanogaster
Abstract
Background: Seven-transmembrane receptors typically mediate olfactory signal transduction by coupling to G-proteins. Although insect odorant receptors have seven transmembrane domains like G-protein coupled receptors, they have an inverted membrane topology and function as ligand-gated cation channels. Consequently, the involvement of cyclic nucleotides and G proteins in insect odor reception is controversial. Since the heterotrimeric Goalpha subunit is expressed in Drosophila olfactory receptor neurons, we reasoned that Go acts together with insect odorant receptor cation channels to mediate odor-induced physiological responses.
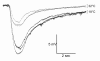
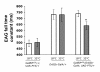
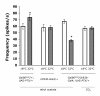
Results: To test whether Go dependent signaling is involved in mediating olfactory responses in Drosophila, we analyzed electroantennogram and single-sensillum recording from flies that conditionally express pertussis toxin, a specific inhibitor of Go in Drosophila. Pertussis toxin expression in olfactory receptor neurons reversibly reduced the amplitude and hastened the termination of electroantennogram responses induced by ethyl acetate. The frequency of odor-induced spike firing from individual sensory neurons was also reduced by pertussis toxin. These results demonstrate that Go signaling is involved in increasing sensitivity of olfactory physiology in Drosophila. The effect of pertussis toxin was independent of odorant identity and intensity, indicating a generalized involvement of Go in olfactory reception.
Conclusion: These results demonstrate that Go is required for maximal physiological responses to multiple odorants in Drosophila, and suggest that OR channel function and G-protein signaling are required for optimal physiological responses to odors.
Figures
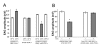
Similar articles
-
Odorant-specific requirements for arrestin function in Drosophila olfaction.J Neurobiol. 2005 Apr;63(1):15-28. doi: 10.1002/neu.20113. J Neurobiol. 2005. PMID: 15627264
-
Role of Go/i subgroup of G proteins in olfactory signaling of Drosophila melanogaster.Eur J Neurosci. 2014 Apr;39(8):1245-55. doi: 10.1111/ejn.12481. Epub 2014 Jan 20. Eur J Neurosci. 2014. PMID: 24443946 Free PMC article.
-
Olfactory maps and odor images.Curr Opin Neurobiol. 2002 Aug;12(4):387-92. doi: 10.1016/s0959-4388(02)00348-3. Curr Opin Neurobiol. 2002. PMID: 12139985 Review.
-
Odorant concentration differentiator for intermittent olfactory signals.J Neurosci. 2014 Dec 10;34(50):16581-93. doi: 10.1523/JNEUROSCI.2319-14.2014. J Neurosci. 2014. PMID: 25505311 Free PMC article.
-
Molecular and cellular basis of human olfaction.Chem Biodivers. 2004 Dec;1(12):1857-69. doi: 10.1002/cbdv.200490142. Chem Biodivers. 2004. PMID: 17191824 Review.
Cited by
-
Insect odorant response sensitivity is tuned by metabotropically autoregulated olfactory receptors.PLoS One. 2013;8(3):e58889. doi: 10.1371/journal.pone.0058889. Epub 2013 Mar 12. PLoS One. 2013. PMID: 23554952 Free PMC article.
-
The Two Main Olfactory Receptor Families in Drosophila, ORs and IRs: A Comparative Approach.Front Cell Neurosci. 2018 Aug 30;12:253. doi: 10.3389/fncel.2018.00253. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30214396 Free PMC article. Review.
-
Tuning Insect Odorant Receptors.Front Cell Neurosci. 2018 Apr 5;12:94. doi: 10.3389/fncel.2018.00094. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29674957 Free PMC article.
-
Regulation of olfactory transduction in the orco channel.Front Cell Neurosci. 2011 Oct 17;5:21. doi: 10.3389/fncel.2011.00021. eCollection 2011. Front Cell Neurosci. 2011. PMID: 22022306 Free PMC article. No abstract available.
-
Hawkmoth Pheromone Transduction Involves G-Protein-Dependent Phospholipase Cβ Signaling.eNeuro. 2025 Mar 7;12(3):ENEURO.0376-24.2024. doi: 10.1523/ENEURO.0376-24.2024. Print 2025 Mar. eNeuro. 2025. PMID: 39880675 Free PMC article.
References
-
- Kain P, Chandrashekaran S, Rodrigues V, Hasan G. Drosophila Mutants in Phospholipid Signaling Have Reduced Olfactory Responses as Adults and Larvae. J Neurogenet. 2008. pp. 1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

