Structure-toxicity relationship of phenolic analogs as anti-melanoma agents: an enzyme directed prodrug approach
- PMID: 19944085
- PMCID: PMC2821678
- DOI: 10.1016/j.cbi.2009.11.020
Structure-toxicity relationship of phenolic analogs as anti-melanoma agents: an enzyme directed prodrug approach
Abstract
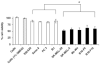
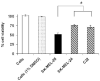
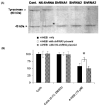
The aim of this study was to identify a phenolic prodrug compound that is minimally metabolized by rat liver microsomes, but yet could form quinone reactive intermediates in melanoma cells as a result of its bioactivation by tyrosinase. In current work, we investigated 24 phenolic compounds for their metabolism by tyrosinase, rat liver microsomes and their toxicity towards murine B16-F0 and human SK-MEL-28 melanoma cells. A linear correlation was found between toxicities of phenolic analogs towards SK-MEL-28 and B16-F0 melanoma cells, suggesting similar mechanisms of toxicity in both cell lines. 4-HEB was identified as the lead compound. 4-HEB (IC(50) 48h, 75muM) showed selective toxicity towards five melanocytic melanoma cell lines SK-MEL-28, SK-MEL-5, MeWo, B16-F0 and B16-F10, which express functional tyrosinase, compared to four non-melanoma cells lines SW-620, Saos-2, PC3 and BJ cells and two amelanotic SK-MEL-24, C32 cells, which do not express functional tyrosinase. 4-HEB caused significant intracellular GSH depletion, ROS formation, and showed significantly less toxicity to tyrosinase specific shRNA transfected SK-MEL-28 cells. Our findings suggest that presence of a phenolic group in 4-HEB is critical for its selective toxicity towards melanoma cells.
Published by Elsevier Ireland Ltd.
Conflict of interest statement
Figures






Similar articles
-
Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines.Chem Biol Interact. 2010 Oct 6;188(1):1-14. doi: 10.1016/j.cbi.2010.05.018. Epub 2010 Jun 4. Chem Biol Interact. 2010. PMID: 20685355 Free PMC article.
-
Biochemical mechanism of acetylsalicylic acid (Aspirin) selective toxicity toward melanoma cell lines.Melanoma Res. 2008 Dec;18(6):386-99. doi: 10.1097/CMR.0b013e3283107df7. Melanoma Res. 2008. PMID: 18971789
-
Efficacy of acetaminophen in skin B16-F0 melanoma tumor-bearing C57BL/6 mice.Int J Oncol. 2009 Jul;35(1):193-204. doi: 10.3892/ijo_00000329. Int J Oncol. 2009. PMID: 19513568
-
Biochemical mechanism of acetaminophen (APAP) induced toxicity in melanoma cell lines.J Pharm Sci. 2009 Apr;98(4):1409-25. doi: 10.1002/jps.21505. J Pharm Sci. 2009. PMID: 18759348 Free PMC article.
-
Exploiting tyrosinase expression and activity in melanocytic tumors: quercetin and the central role of p53.Integr Cancer Ther. 2011 Dec;10(4):328-40. doi: 10.1177/1534735410391661. Epub 2010 Dec 31. Integr Cancer Ther. 2011. PMID: 21196432 Review.
Cited by
-
Biochemical mechanism of caffeic acid phenylethyl ester (CAPE) selective toxicity towards melanoma cell lines.Chem Biol Interact. 2010 Oct 6;188(1):1-14. doi: 10.1016/j.cbi.2010.05.018. Epub 2010 Jun 4. Chem Biol Interact. 2010. PMID: 20685355 Free PMC article.
-
A Disposable Amperometric Sensor Based on High-Performance PEDOT:PSS/Ionic Liquid Nanocomposite Thin Film-Modified Screen-Printed Electrode for the Analysis of Catechol in Natural Water Samples.Sensors (Basel). 2017 Jul 26;17(8):1716. doi: 10.3390/s17081716. Sensors (Basel). 2017. PMID: 28933756 Free PMC article.
-
Depigmenting effect of Kojic acid esters in hyperpigmented B16F1 melanoma cells.J Biomed Biotechnol. 2012;2012:952452. doi: 10.1155/2012/952452. Epub 2012 Oct 2. J Biomed Biotechnol. 2012. PMID: 23091364 Free PMC article.
-
Melanocytotoxic chemicals and their toxic mechanisms.Toxicol Res. 2022 Aug 22;38(4):417-435. doi: 10.1007/s43188-022-00144-2. eCollection 2022 Oct. Toxicol Res. 2022. PMID: 36277364 Free PMC article. Review.
-
New insights into the anti-inflammatory and anti-melanoma mechanisms of action of azelaic acid and other Fusarium solani metabolites via in vitro and in silico studies.Sci Rep. 2024 Jun 22;14(1):14370. doi: 10.1038/s41598-024-63958-0. Sci Rep. 2024. PMID: 38909081 Free PMC article.
References
-
- Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK, editors. SEER Cancer Statistics Review, 1975-2001. National Cancer Institute; Bethesda, MD: 2004. http://seer.cancer.gov/csr/1975_2001/
-
- Anderson CM, Buzaid AC, Legha SS. Systemic treatments for advanced cutaneous melanoma. Oncology (Williston Park) 1995;9(11):1149–1158. discussion 1163-1164, 1167-1168. - PubMed
-
- Riley PA. Hydroxyanisole depigmentation: In-vitro studies. J Pathol. 1969;97(2):193–206. - PubMed
-
- Ojima I. Guided molecular missiles for tumor-targeting chemotherapy--case studies using the second-generation taxoids as warheads. Acc Chem Res. 2008;41(1):108–119. - PubMed
-
- Vielkind U, Schlage W, Anders F. Melanogenesis in genetically determined pigment cell tumors of platyfish and platyfish-swordtail hybrids: correlation between tyrosine activity and degree of malignancy. Cancer Res Clin Oncol. 1977;90(3):285–299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

