Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies
- PMID: 19944097
- PMCID: PMC2812599
- DOI: 10.1016/j.expneurol.2009.11.015
Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies
Abstract
Alpha-synuclein (alpha-syn) aggregation is a neuropathological hallmark of many diseases including Dementia with Lewy Bodies (DLB) and Parkinson's Disease (PD), collectively termed the alpha-synucleinopathies. The mechanisms underlying alpha-syn aggregation remain elusive though emerging science has hypothesized that the interaction between cholesterol and alpha-syn may play a role. Cholesterol has been linked to alpha-synucleinopathies by recent work suggesting cholesterol metabolites appear to accelerate alpha-syn fibrillization. Consistent with these findings, cholesterol-lowering agents have been demonstrated to reduce alpha-syn accumulation and the associated neuronal pathology in vitro. In this context, this study sought to investigate the in vivo effects of the cholesterol synthesis inhibitor lovastatin on alpha-syn aggregation in two different transgenic (Tg) mouse models that neuronally overexpress human alpha-syn. Lovastatin-treated mice displayed significantly reduced plasma cholesterol levels and levels of oxidized cholesterol metabolites in the brain in comparison to saline-treated controls. Immunohistochemical analysis demonstrated a significant reduction of neuronal alpha-syn aggregates and alpha-syn immunoreactive neuropil in the temporal cortex of lovastatin-treated Tg mice in comparison to saline-treated alpha-syn Tg controls. Consistently, immunoblot analysis of mouse brain homogenates showed a reduction in levels of total and oxidized alpha-syn in lovastatin-treated alpha-syn Tg mice in comparison to saline-treated alpha-syn Tg controls. The reduced alpha-syn accumulation in lovastatin-treated mice was associated with abrogation of neuronal pathology. The results from this study demonstrate that lovastatin administration can reduce alpha-syn aggregation and associated neuropathology and support the possibility that treatment with cholesterol-lowering agents may be beneficial for patients with PD and/or DLB.
Copyright 2009 Elsevier Inc. All rights reserved.
Figures
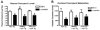
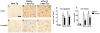
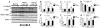
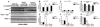
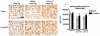

Similar articles
-
Neurotoxic conversion of beta-synuclein: a novel approach to generate a transgenic mouse model of synucleinopathies?J Neurol. 2009 Aug;256 Suppl 3:286-92. doi: 10.1007/s00415-009-5246-8. J Neurol. 2009. PMID: 19711118 Review.
-
Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson's disease.J Neurochem. 2008 Jun;105(5):1656-67. doi: 10.1111/j.1471-4159.2008.05254.x. Epub 2008 Jan 28. J Neurochem. 2008. PMID: 18248604 Free PMC article.
-
Alterations in mGluR5 expression and signaling in Lewy body disease and in transgenic models of alpha-synucleinopathy--implications for excitotoxicity.PLoS One. 2010 Nov 16;5(11):e14020. doi: 10.1371/journal.pone.0014020. PLoS One. 2010. Retraction in: PLoS One. 2024 Nov 14;19(11):e0314138. doi: 10.1371/journal.pone.0314138. PMID: 21103359 Free PMC article. Retracted.
-
Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson's and Lewy body diseases.J Neurosci. 2009 Oct 28;29(43):13578-88. doi: 10.1523/JNEUROSCI.4390-09.2009. J Neurosci. 2009. PMID: 19864570 Free PMC article.
-
The contribution of alpha synuclein to neuronal survival and function - Implications for Parkinson's disease.J Neurochem. 2016 May;137(3):331-59. doi: 10.1111/jnc.13570. Epub 2016 Mar 23. J Neurochem. 2016. PMID: 26852372 Free PMC article. Review.
Cited by
-
The Aggregation of Huntingtin and α-Synuclein.J Biophys. 2012;2012:606172. doi: 10.1155/2012/606172. Epub 2012 Jul 26. J Biophys. 2012. PMID: 22899913 Free PMC article.
-
Parkinson's disease mouse models in translational research.Mamm Genome. 2011 Aug;22(7-8):401-19. doi: 10.1007/s00335-011-9330-x. Epub 2011 May 11. Mamm Genome. 2011. PMID: 21559878 Free PMC article. Review.
-
Potential Crosstalk Between Parkinson's Disease and Energy Metabolism.Aging Dis. 2021 Dec 1;12(8):2003-2015. doi: 10.14336/AD.2021.0422. eCollection 2021 Dec. Aging Dis. 2021. PMID: 34881082 Free PMC article. Review.
-
Prospects of statins in Parkinson disease.Neuroscientist. 2011 Jun;17(3):244-55. doi: 10.1177/1073858410385006. Epub 2011 Jan 20. Neuroscientist. 2011. PMID: 21252380 Free PMC article. Review.
-
Transcript expression levels of full-length alpha-synuclein and its three alternatively spliced variants in Parkinson's disease brain regions and in a transgenic mouse model of alpha-synuclein overexpression.Mol Cell Neurosci. 2012 Feb;49(2):230-9. doi: 10.1016/j.mcn.2011.11.006. Epub 2011 Dec 6. Mol Cell Neurosci. 2012. PMID: 22155155 Free PMC article.
References
-
- Alberts AW, Chen J, Kuron G, Hunt V, Huff J, Hoffman C, Rothrock J, Lopez M, Joshua H, Harris E, Patchett A, Monaghan R, Currie S, Stapley E, Albers-Schonberg G, Hensens O, Hirshfield J, Hoogsteen K, Liesch J, Springer J. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci U S A. 1980;77:3957–3961. - PMC - PubMed
-
- Bar-On P, Rockenstein E, Adame A, Ho G, Hashimoto M, Masliah E. Effects of the cholesterol-lowering compound methyl-beta-cyclodextrin in models of alpha-synucleinopathy. J Neurochem. 2006;98:1032–1045. - PubMed
-
- Bieschke J, Zhang Q, Bosco DA, Lerner RA, Powers ET, Wentworth P, Jr., Kelly JW. Small molecule oxidation products trigger disease-associated protein misfolding. Acc Chem Res. 2006;39:611–619. - PubMed
-
- Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG10435/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- NS44233/NS/NINDS NIH HHS/United States
- AG022074/AG/NIA NIH HHS/United States
- P01 NS044233/NS/NINDS NIH HHS/United States
- P01 ES016738/ES/NIEHS NIH HHS/United States
- AG5131/AG/NIA NIH HHS/United States
- P41 RR004050/RR/NCRR NIH HHS/United States
- R01 AG018440/AG/NIA NIH HHS/United States
- R37 AG018440/AG/NIA NIH HHS/United States
- P01 AG010435/AG/NIA NIH HHS/United States
- AG18440/AG/NIA NIH HHS/United States
- P01 AG022074/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

