A host-specific factor is necessary for efficient folding of the autotransporter plasmid-encoded toxin
- PMID: 19944129
- PMCID: PMC2823069
- DOI: 10.1016/j.biochi.2009.11.006
A host-specific factor is necessary for efficient folding of the autotransporter plasmid-encoded toxin
Abstract
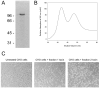
Autotransporters are the most common virulence factors secreted from Gram-negative pathogens. Until recently, autotransporter folding and outer membrane translocation were thought to be self-mediated events that did not require accessory factors. Here, we report that two variants of the autotransporter plasmid-encoded toxin are secreted by a lab strain of Escherichia coli. Biophysical analysis and cell-based toxicity assays demonstrated that only one of the two variants was in a folded, active conformation. The misfolded variant was not produced by a pathogenic strain of enteroaggregative E. coli and did not result from protein overproduction in the lab strain of E. coli. Our data suggest a host-specific factor is required for efficient folding of plasmid-encoded toxin.
2009 Elsevier Masson SAS. All rights reserved.
Figures






References
-
- Dautin N, Bernstein HD. Protein secretion in gram-negative bacteria via the autotransporter pathway. Annu Rev Microbiol. 2007;61:89–112. - PubMed
-
- Yen YT, Kostakioti M, Henderson IR, Stathopoulos C. Common themes and variations in serine protease autotransporters. Trends Microbiol. 2008;16:370–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

