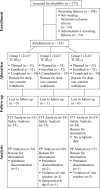
A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE
- PMID: 19944151
- PMCID: PMC2814951
- DOI: 10.1016/j.vaccine.2009.11.030
A randomized, double-blind, dose-finding Phase II study to evaluate immunogenicity and safety of the third generation smallpox vaccine candidate IMVAMUNE
Abstract
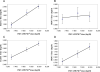
IMVAMUNE is a Modified Vaccinia Ankara (MVA)-based virus that is being developed as a safer 3rd generation smallpox vaccine. In order to determine the optimal dose for further development, a double-blind, randomized Phase II trial was performed testing three different doses of IMVAMUNE in 164 healthy volunteers. All three IMVAMUNE doses displayed a favourable safety profile, with local reactions as the most frequent observation. The 1 x 10(8)TCID(50) IMVAMUNE dose induced a total antibody response in 94% of the subjects following the first vaccination and the highest peak seroconversion rates by ELISA (100%) and PRNT (71%). This IMVAMUNE dose was considered to be optimal for the further clinical development of this highly attenuated poxvirus as a safer smallpox vaccine.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures
References
-
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. WHO. Geneva, Switzerland: 1988. Smallpox and its eradication. whqlibdoc.who.int/smallpox/9241561106.pdf.
-
- Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination, 1968. N Engl J Med. 1969;281(22):1201–8. - PubMed
-
- Lane JM, Ruben FL, Abrutyn E, Millar JD. Deaths attributable to smallpox vaccination, 1959 to 1966, and 1968. JAMA. 1970;212(3):441–4. - PubMed
-
- Grabenstein JD, Winkenwerder W., Jr. US military smallpox vaccination program experience. JAMA. 2003;289(24):3278–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

