Loss-of-function mutations in the human ortholog of Chlamydomonas reinhardtii ODA7 disrupt dynein arm assembly and cause primary ciliary dyskinesia
- PMID: 19944405
- PMCID: PMC2790569
- DOI: 10.1016/j.ajhg.2009.11.008
Loss-of-function mutations in the human ortholog of Chlamydomonas reinhardtii ODA7 disrupt dynein arm assembly and cause primary ciliary dyskinesia
Abstract
Cilia and flagella are evolutionarily conserved structures that play various physiological roles in diverse cell types. Defects in motile cilia result in primary ciliary dyskinesia (PCD), the most prominent ciliopathy, characterized by the association of respiratory symptoms, male infertility, and, in nearly 50% of cases, situs inversus. So far, most identified disease-causing mutations involve genes encoding various ciliary components, such those belonging to the dynein arms that are essential for ciliary motion. Following a candidate-gene approach based on data from a mutant strain of the biflagellated alga Chlamydomonas reinhardtii carrying an ODA7 defect, we identified four families with a PCD phenotype characterized by the absence of both dynein arms and loss-of-function mutations in the human orthologous gene called LRRC50. Functional analyses performed in Chlamydomonas reinhardtii and in another flagellated protist, Trypanosoma brucei, support a key role for LRRC50, a member of the leucine-rich-repeat superfamily, in cytoplasmic preassembly of dynein arms.
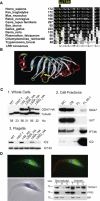
Figures


References
-
- Satir P., Christensen S.T. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol. 2007;69:377–400. - PubMed
-
- Basu B., Brueckner M. Cilia multifunctional organelles at the center of vertebrate left-right asymmetry. Curr. Top. Dev. Biol. 2008;85:151–174. - PubMed
-
- Afzelius B.A. A human syndrome caused by immotile cilia. Science. 1976;193:317–319. - PubMed
-
- Duriez B., Duquesnoy P., Escudier E., Bridoux A.M., Escalier D., Rayet I., Marcos E., Vojtek A.M., Bercher J.F., Amselem S. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc. Natl. Acad. Sci. USA. 2007;104:3336–3341. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

