The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells
- PMID: 19944458
- PMCID: PMC2813971
- DOI: 10.1016/j.biomaterials.2009.10.019
The role of organ level conditioning on the promotion of engineered heart valve tissue development in-vitro using mesenchymal stem cells
Abstract
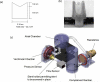
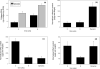
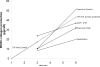
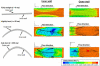
We have previously shown that combined flexure and flow (CFF) augment engineered heart valve tissue formation using bone marrow-derived mesenchymal stem cells (MSC) seeded on polyglycolic acid (PGA)/poly-L-lactic acid (PLLA) blend nonwoven fibrous scaffolds (Engelmayr, et al., Biomaterials 2006; vol. 27 pp. 6083-95). In the present study, we sought to determine if these phenomena were reproducible at the organ level in a functional tri-leaflet valve. Tissue engineered valve constructs (TEVC) were fabricated using PGA/PLLA nonwoven fibrous scaffolds then seeded with MSCs. Tissue formation rates using both standard and augmented (using basic fibroblast growth factor [bFGF] and ascorbic acid-2-phosphate [AA2P]) media to enhance the overall production of collagen were evaluated, along with their relation to the local fluid flow fields. The resulting TEVCs were statically cultured for 3 weeks, followed by a 3 week dynamic culture period using our organ level bioreactor (Hildebrand et al., ABME, Vol. 32, pp. 1039-49, 2004) under approximated pulmonary artery conditions. Results indicated that supplemented media accelerated collagen formation (approximately 185% increase in collagen mass/MSC compared to standard media), as well as increasing collagen mass production from 3.90 to 4.43 pg/cell/week from 3 to 6 weeks. Using augmented media, dynamic conditioning increased collagen mass production rate from 7.23 to 13.65 pg/cell/week (88.8%) during the dynamic culture period, along with greater preservation of net DNA. Moreover, when compared to our previous CFF study, organ level conditioning increased the collagen production rate from 4.76 to 6.42 pg/cell/week (35%). Newly conducted CFD studies of the CFF specimen flow patterns suggested that oscillatory surface shear stresses were surprisingly similar to a tri-leaflet valve. Overall, we found that the use of simulated pulmonary artery conditions resulted in substantially larger collagen mass production levels and rates found in our earlier CFF study. Moreover, given the fact that the scaffolds underwent modest strains (approximately 7% max) during either CFF or physiological conditioning, the oscillatory surface shear stresses estimated in both studies may play a substantial role in eliciting MSC collagen production in the highly dynamic engineered heart valve fluid mechanical environment.
(c) 2009 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
A novel bioreactor for mechanobiological studies of engineered heart valve tissue formation under pulmonary arterial physiological flow conditions.J Biomech Eng. 2014 Dec;136(12):121009. doi: 10.1115/1.4028815. J Biomech Eng. 2014. PMID: 25321615 Free PMC article.
-
Cyclic flexure and laminar flow synergistically accelerate mesenchymal stem cell-mediated engineered tissue formation: Implications for engineered heart valve tissues.Biomaterials. 2006 Dec;27(36):6083-95. doi: 10.1016/j.biomaterials.2006.07.045. Epub 2006 Aug 23. Biomaterials. 2006. PMID: 16930686
-
Computational simulations predict a key role for oscillatory fluid shear stress in de novo valvular tissue formation.J Biomech. 2014 Nov 7;47(14):3517-23. doi: 10.1016/j.jbiomech.2014.08.028. Epub 2014 Sep 16. J Biomech. 2014. PMID: 25262874
-
Biomechanical conditioning of tissue engineered heart valves: Too much of a good thing?Adv Drug Deliv Rev. 2016 Jan 15;96:161-75. doi: 10.1016/j.addr.2015.11.003. Epub 2015 Nov 10. Adv Drug Deliv Rev. 2016. PMID: 26555371 Review.
-
Cells for tissue engineering of cardiac valves.J Tissue Eng Regen Med. 2016 Oct;10(10):804-824. doi: 10.1002/term.2010. Epub 2015 Feb 25. J Tissue Eng Regen Med. 2016. PMID: 25712485 Review.
Cited by
-
Periodontal ligament cells cultured under steady-flow environments demonstrate potential for use in heart valve tissue engineering.Tissue Eng Part A. 2013 Feb;19(3-4):458-66. doi: 10.1089/ten.TEA.2012.0149. Epub 2012 Oct 19. Tissue Eng Part A. 2013. PMID: 22958144 Free PMC article.
-
A triphasic constrained mixture model of engineered tissue formation under in vitro dynamic mechanical conditioning.Biomech Model Mechanobiol. 2016 Apr;15(2):293-316. doi: 10.1007/s10237-015-0687-8. Epub 2015 Jun 9. Biomech Model Mechanobiol. 2016. PMID: 26055347 Free PMC article.
-
Mesenchymal Stem Cell Seeding of Porcine Small Intestinal Submucosal Extracellular Matrix for Cardiovascular Applications.PLoS One. 2016 Apr 12;11(4):e0153412. doi: 10.1371/journal.pone.0153412. eCollection 2016. PLoS One. 2016. PMID: 27070546 Free PMC article.
-
Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds.Biofabrication. 2012 Sep;4(3):035005. doi: 10.1088/1758-5082/4/3/035005. Epub 2012 Aug 23. Biofabrication. 2012. PMID: 22914604 Free PMC article.
-
A novel bioreactor for mechanobiological studies of engineered heart valve tissue formation under pulmonary arterial physiological flow conditions.J Biomech Eng. 2014 Dec;136(12):121009. doi: 10.1115/1.4028815. J Biomech Eng. 2014. PMID: 25321615 Free PMC article.
References
-
- Mayer JE, Jr., Shin'oka T, Shum-Tim D. Tissue engineering of cardiovascular structures. Curr Opin Cardiol. 1997;12(6):528–32. - PubMed
-
- Sacks MS, Schoen FJ, Mayer JE., Jr Bioengineering Challenges for Heart Valve Tissue Engineering. Annual Review of Biomedical Engineering. 2009:11. - PubMed
-
- Dumont K, Yperman J, Verbeken E, Segers P, Meuris B, Vandenberghe S, et al. Design of a new pulsatile bioreactor for tissue engineered aortic heart valve formation. Artif Organs. 2002;26(8):710–4. - PubMed
-
- Engelmayr GC, Jr., Rabkin E, Sutherland FW, Schoen FJ, Mayer JE, Jr., Sacks MS. The independent role of cyclic flexure in the early in vitro development of an engineered heart valve tissue. Biomaterials. 2005;26(2):175–87. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

