miR-9a prevents apoptosis during wing development by repressing Drosophila LIM-only
- PMID: 19944676
- PMCID: PMC2812678
- DOI: 10.1016/j.ydbio.2009.11.025
miR-9a prevents apoptosis during wing development by repressing Drosophila LIM-only
Abstract
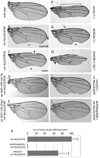
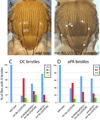
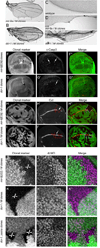
Loss of Drosophila mir-9a induces a subtle increase in sensory bristles, but a substantial loss of wing tissue. Here, we establish that the latter phenotype is largely due to ectopic apoptosis in the dorsal wing primordium, and we could rescue wing development in the absence of this microRNA by dorsal-specific inhibition of apoptosis. Such apoptosis was a consequence of de-repressing Drosophila LIM-only (dLMO), which encodes a transcriptional regulator of wing and neural development. We observed cell-autonomous elevation of endogenous dLMO and a GFP-dLMO 3'UTR sensor in mir-9a mutant wing clones, and heterozygosity for dLMO rescued the apoptosis and wing defects of mir-9a mutants. We also provide evidence that dLMO, in addition to senseless, contributes to the bristle defects of the mir-9a mutant. Unexpectedly, the upregulation of dLMO, loss of Cut, and adult wing margin defects seen with mir-9a mutant clones were not recapitulated by clonal loss of the miRNA biogenesis factors Dicer-1 or Pasha, even though these mutant conditions similarly de-repressed miR-9a and dLMO sensor transgenes. Therefore, the failure to observe a phenotype upon conditional knockout of a miRNA processing factor does not reliably indicate the lack of critical roles of miRNAs in a given setting.
Figures
References
-
- Aravin A, Lagos-Quintana M, Yalcin A, Zavolan M, Marks D, Snyder B, Gaasterland T, Meyer J, Tuschl T. The small RNA profile during Drosophila melanogaster development. Dev. Cell. 2003;5:337–350. - PubMed
-
- Asmar J, Biryukova I, Heitzler P. Drosophila dLMO-PA isoform acts as an early activator of achaete/scute proneural expression. Dev Biol. 2008;316:487–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

