Genomic variation and neurohormonal intervention in heart failure
- PMID: 19945059
- PMCID: PMC3421049
- DOI: 10.1016/j.hfc.2009.08.004
Genomic variation and neurohormonal intervention in heart failure
Abstract
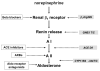
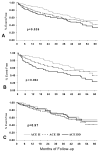
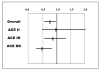
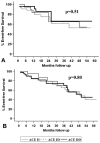
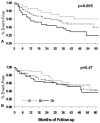
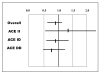
Neurohormonal activation is an important driver of heart-failure progression, and all pharmacologic interventions that improve heart-failure survival inhibit this systemic response to myocardial injury. Adrenergic stimulation of beta(1) receptors in the kidney results in the release of plasma renin, the conversion of peptide precursors to angiotensin II (a2), and ultimately the production of aldosterone. beta(1)-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), and aldosterone receptor antagonists all act by inhibiting the activity of critical protein of this core pathway: the beta(1) receptor, ACE, the a2 receptor, and aldosterone synthase. Investigation of the pharmacogenetic interactions of the ACE D/I polymorphism and heart-failure therapy demonstrates the power of genomics to target therapeutics. This review explores how genetic variation in genes involved in neurohormonal activation influences heart-failure outcomes and the impact of pharmacotherapy.
Figures
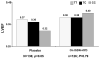
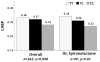
References
-
- Osler W. The principles and practice of medicine. D. Appleton and Co.; New York: 1892. pp. 623–640.
-
- Danser AH, Derkx FH, Hense HW, Jeunemaitre X, Riegger GA, Schunkert H. Angiotensinogen (M235T) and angiotensin-converting enzyme (I/D) polymorphisms in association with plasma renin and prorenin levels. J Hypertens. 1998;16:1879–1883. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

