TFB2 is a transient component of the catalytic site of the human mitochondrial RNA polymerase
- PMID: 19945377
- PMCID: PMC2806307
- DOI: 10.1016/j.cell.2009.10.031
TFB2 is a transient component of the catalytic site of the human mitochondrial RNA polymerase
Abstract
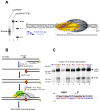
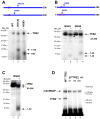
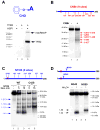
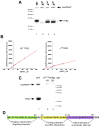
Transcription in human mitochondria is carried out by a single-subunit, T7-like RNA polymerase assisted by several auxiliary factors. We demonstrate that an essential initiation factor, TFB2, forms a network of interactions with DNA near the transcription start site and facilitates promoter melting but may not be essential for promoter recognition. Unexpectedly, catalytic autolabeling reveals that TFB2 interacts with the priming substrate, suggesting that TFB2 acts as a transient component of the catalytic site of the initiation complex. Mapping of TFB2 identifies a region of its N-terminal domain that is involved in simultaneous interactions with the priming substrate and the templating (+1) DNA base. Our data indicate that the transcriptional machinery in human mitochondria has evolved into a system that combines features inherited from self-sufficient, T7-like RNA polymerase and those typically found in systems comprising cellular multi-subunit polymerases, and provide insights into the molecular mechanisms of transcription regulation in mitochondria.
Figures


Similar articles
-
A novel intermediate in transcription initiation by human mitochondrial RNA polymerase.Nucleic Acids Res. 2014 Apr;42(6):3884-93. doi: 10.1093/nar/gkt1356. Epub 2014 Jan 6. Nucleic Acids Res. 2014. PMID: 24393772 Free PMC article.
-
Human mitochondrial RNA polymerase structures reveal transcription start site and slippage mechanism.Mol Cell. 2025 Aug 21;85(16):3137-3150.e7. doi: 10.1016/j.molcel.2025.07.002. Epub 2025 Jul 24. Mol Cell. 2025. PMID: 40712586
-
Structure of human mitochondrial RNA polymerase.Nature. 2011 Sep 25;478(7368):269-73. doi: 10.1038/nature10435. Nature. 2011. PMID: 21947009
-
Mechanism of transcription initiation by the yeast mitochondrial RNA polymerase.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):930-8. doi: 10.1016/j.bbagrm.2012.02.003. Epub 2012 Feb 14. Biochim Biophys Acta. 2012. PMID: 22353467 Free PMC article. Review.
-
Mitochondrial transcription and its regulation in mammalian cells.Trends Biochem Sci. 2007 Mar;32(3):111-7. doi: 10.1016/j.tibs.2007.01.003. Epub 2007 Feb 8. Trends Biochem Sci. 2007. PMID: 17291767 Review.
Cited by
-
Dynamic regulation of mitochondrial transcription as a mechanism of cellular adaptation.Biochim Biophys Acta. 2012 Sep-Oct;1819(9-10):1075-9. doi: 10.1016/j.bbagrm.2012.06.004. Epub 2012 Jul 3. Biochim Biophys Acta. 2012. PMID: 22766037 Free PMC article. Review.
-
Structural basis of mitochondrial transcription.Nat Struct Mol Biol. 2018 Sep;25(9):754-765. doi: 10.1038/s41594-018-0122-9. Epub 2018 Sep 6. Nat Struct Mol Biol. 2018. PMID: 30190598 Free PMC article. Review.
-
Mitochondrial Transcription of Entomopathogenic Fungi Reveals Evolutionary Aspects of Mitogenomes.Front Microbiol. 2022 Mar 21;13:821638. doi: 10.3389/fmicb.2022.821638. eCollection 2022. Front Microbiol. 2022. PMID: 35387072 Free PMC article.
-
Mitochondrial DNA mutations in disease and aging.J Cell Biol. 2011 May 30;193(5):809-18. doi: 10.1083/jcb.201010024. Epub 2011 May 23. J Cell Biol. 2011. PMID: 21606204 Free PMC article. Review.
-
Mitochondrial transcription factor B2 is essential for mitochondrial and cellular function in pancreatic β-cells.Mol Metab. 2017 May 19;6(7):651-663. doi: 10.1016/j.molmet.2017.05.005. eCollection 2017 Jul. Mol Metab. 2017. PMID: 28702322 Free PMC article.
References
-
- Amiott EA, Jaehning JA. Mitochondrial transcription is regulated via an ATP “sensing” mechanism that couples RNA abundance to respiration. Mol Cell. 2006a;22:329–338. - PubMed
-
- Amiott EA, Jaehning JA. Sensitivity of the yeast mitochondrial RNA polymerase to +1 and +2 initiating nucleotides. J Biol Chem. 2006b;281:34982–34988. - PubMed
-
- Bonawitz ND, Clayton DA, Shadel GS. Initiation and beyond: multiple functions of the human mitochondrial transcription machinery. Mol Cell. 2006;24:813–825. - PubMed
-
- Chang DD, Clayton DA. Precise identification of individual promoters for transcription of each strand of human mitochondrial DNA. Cell. 1984;36:635–643. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

