Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112
- PMID: 19945794
- PMCID: PMC2861283
- DOI: 10.1016/j.pain.2009.10.026
Long-lasting antinociceptive spinal effects in primates of the novel nociceptin/orphanin FQ receptor agonist UFP-112
Abstract
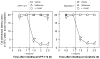
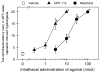
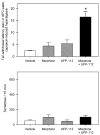
Chemical modifications of nociceptin/orphanin FQ (N/OFQ) peptide that result in increased potency and resistance to degradation have recently lead to the discovery of [(pF)Phe(4)Aib(7)Arg(14)Lys(15)]N/OFQ-NH(2) (UFP-112), a novel N/OFQ peptide (NOP) receptor agonist. The aim of this study was to investigate the pharmacological profile of intrathecally administered UFP-112 in monkeys under different behavioral assays. Intrathecal UFP-112 (1-10 nmol) dose-dependently produced antinociception against an acute noxious stimulus (50 degrees C water) and capsaicin-induced thermal hyperalgesia. Intrathecal UFP-112-induced antinociception could be reversed by a NOP receptor antagonist, J-113397 (0.1mg/kg), but not by a classic opioid receptor antagonist, naltrexone (0.03 mg/kg). Like intrathecal morphine, UFP-112 produced antinociception in two primate pain models with a similar magnitude of effectiveness and a similar duration of action that last for 4-5h. Unlike intrathecal morphine, UFP-112 did not produce itch/scratching responses. In addition, intrathecal inactive doses of UFP-112 and morphine produced significant antinociceptive effects when given in combination without increasing scratching responses. These results demonstrated that intrathecal UFP-112 produced long-lasting morphine-comparable antinociceptive effects without potential itch side effect. This study is the first to provide functional evidence that selective NOP receptor agonists such as UFP-112 alone or in conjunction with morphine may improve the quality of spinal analgesia.
Copyright 2009 International Association for the Study of Pain. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
All authors declare that they have no conflicts of interest.
Figures



Similar articles
-
Antinociceptive effects of nociceptin/orphanin FQ administered intrathecally in monkeys.J Pain. 2009 May;10(5):509-16. doi: 10.1016/j.jpain.2008.11.006. Epub 2009 Feb 23. J Pain. 2009. PMID: 19231294 Free PMC article.
-
Acute and subchronic antinociceptive effects of nociceptin/orphanin FQ receptor agonists infused by intrathecal route in rats.Eur J Pharmacol. 2015 May 5;754:73-81. doi: 10.1016/j.ejphar.2015.02.020. Epub 2015 Feb 19. Eur J Pharmacol. 2015. PMID: 25704616
-
Effects of intrathecally administered nociceptin/orphanin FQ in monkeys: behavioral and mass spectrometric studies.J Pharmacol Exp Ther. 2006 Sep;318(3):1257-64. doi: 10.1124/jpet.106.106120. Epub 2006 Jun 9. J Pharmacol Exp Ther. 2006. PMID: 16766718
-
UFP-112 a potent and long-lasting agonist selective for the Nociceptin/Orphanin FQ receptor.CNS Neurosci Ther. 2011 Jun;17(3):178-98. doi: 10.1111/j.1755-5949.2009.00107.x. Epub 2010 May 18. CNS Neurosci Ther. 2011. PMID: 20497197 Free PMC article. Review.
-
Nociceptin/orphanin FQ peptide receptors: pharmacology and clinical implications.Curr Drug Targets. 2007 Jan;8(1):117-35. doi: 10.2174/138945007779315605. Curr Drug Targets. 2007. PMID: 17266536 Review.
Cited by
-
Oxycodone in the Opioid Epidemic: High 'Liking', 'Wanting', and Abuse Liability.Cell Mol Neurobiol. 2021 Jul;41(5):899-926. doi: 10.1007/s10571-020-01013-y. Epub 2020 Nov 27. Cell Mol Neurobiol. 2021. PMID: 33245509 Free PMC article. Review.
-
Opioid Analgesia and Opioid-Induced Adverse Effects: A Review.Pharmaceuticals (Basel). 2021 Oct 27;14(11):1091. doi: 10.3390/ph14111091. Pharmaceuticals (Basel). 2021. PMID: 34832873 Free PMC article. Review.
-
Neuraxial opioid-induced itch and its pharmacological antagonism.Handb Exp Pharmacol. 2015;226:315-35. doi: 10.1007/978-3-662-44605-8_17. Handb Exp Pharmacol. 2015. PMID: 25861787 Free PMC article. Review.
-
Nociceptin Opioid Receptor (NOP) as a Therapeutic Target: Progress in Translation from Preclinical Research to Clinical Utility.J Med Chem. 2016 Aug 11;59(15):7011-28. doi: 10.1021/acs.jmedchem.5b01499. Epub 2016 Mar 14. J Med Chem. 2016. PMID: 26878436 Free PMC article. Review.
-
Spinal antinociceptive effects of the novel NOP receptor agonist PWT2-nociceptin/orphanin FQ in mice and monkeys.Br J Pharmacol. 2015 Jul;172(14):3661-70. doi: 10.1111/bph.13150. Epub 2015 May 12. Br J Pharmacol. 2015. PMID: 25828800 Free PMC article.
References
-
- Arduin M, Spagnolo B, Calo G, Guerrini R, Carra G, Fischetti C, Trapella C, Marzola E, McDonald J, Lambert DG, Regoli D, Salvadori S. Synthesis and biological activity of nociceptin/orphanin FQ analogues substituted in position 7 or 11 with Cα,α-dialkylated amino acids. Bioorg Med Chem. 2007;15:4434–43. - PubMed
-
- Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, Stanley TH. Dose–response pharmacology of intrathecal morphine in human volunteers. Anesthesiology. 1993;79:49–59. - PubMed
-
- Baraka A, Noueihid R, Hajj S. Intrathecal injection of morphine for obstetric analgesia. Anesthesiology. 1981;54:136–40. - PubMed
-
- Buerkle H, Yaksh TL. Comparison of the spinal actions of the mu-opioid remifentanil with alfentanil and morphine in the rat. Anesthesiology. 1996;84:94–102. - PubMed
-
- Butelman ER, Harris TJ, Kreek MJ. Antiallodynic effects of loperamide and fentanyl against topical capsaicin-induced allodynia in unanesthetized primates. J Pharmacol Exp Ther. 2004;311:155–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

