The peptide hormone pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites
- PMID: 19946074
- PMCID: PMC2784736
- DOI: 10.1242/jeb.035741
The peptide hormone pQDLDHVFLRFamide (crustacean myosuppressin) modulates the Homarus americanus cardiac neuromuscular system at multiple sites
Abstract
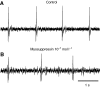
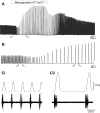
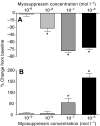
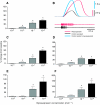
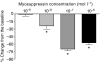
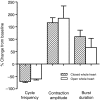
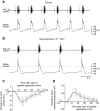
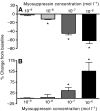
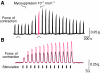
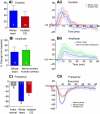
pQDLDHVFLRFamide is a highly conserved crustacean neuropeptide with a structure that places it within the myosuppressin subfamily of the FMRFamide-like peptides. Despite its apparent ubiquitous conservation in decapod crustaceans, the paracrine and/or endocrine roles played by pQDLDHVFLRFamide remain largely unknown. We have examined the actions of this peptide on the cardiac neuromuscular system of the American lobster Homarus americanus using four preparations: the intact animal, the heart in vitro, the isolated cardiac ganglion (CG), and a stimulated heart muscle preparation. In the intact animal, injection of myosuppressin caused a decrease in heartbeat frequency. Perfusion of the in vitro heart with pQDLDHVFLRFamide elicited a decrease in the frequency and an increase in the amplitude of heart contractions. In the isolated CG, myosuppressin induced a hyperpolarization of the resting membrane potential of cardiac motor neurons and a decrease in the cycle frequency of their bursting. In the stimulated heart muscle preparation, pQDLDHVFLRFamide increased the amplitude of the induced contractions, suggesting that myosuppressin modulates not only the CG, but also peripheral sites. For at least the in vitro heart and the isolated CG, the effects of myosuppressin were dose-dependent (10(-9) to 10(-6) mol l(-1) tested), with threshold concentrations (10(-8)-10(-7) mol l(-1)) consistent with the peptide serving as a circulating hormone. Although cycle frequency, a parameter directly determined by the CG, consistently decreased when pQDLDHVFLRFamide was applied to all preparation types, the magnitudes of this decrease differed, suggesting the possibility that, because myosuppressin modulates the CG and the periphery, it also alters peripheral feedback to the CG.
Figures

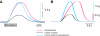
References
-
- Alexandrowicz J. S. (1932). Innervation of the heart of the Crustacea. I. Decapoda. Q. J. Microsc. Sci. 75, 182-249
-
- Anderson M., Cooke I. M. (1971). Neural activation of the heart of the lobster Homarus americanus. J. Exp. Biol. 55, 449-468 - PubMed
-
- Battelle B. A., Kravitz E. A. (1978). Targets of octopamine action in the lobster: cyclic nucleotide changes and physiological effects in hemolymph, heart and exoskeletal muscle. J. Pharmacol. Exp. Ther. 205, 438-448 - PubMed
-
- Bendtsen J. D., Nielsen H., von Heijne G., Brunak S. (2004). Improved prediction of signal peptides: SignalP 3. 0. J. Mol. Biol. 340, 783-795 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

