Mapping the tree of life: progress and prospects
- PMID: 19946133
- PMCID: PMC2786576
- DOI: 10.1128/MMBR.00033-09
Mapping the tree of life: progress and prospects
Abstract
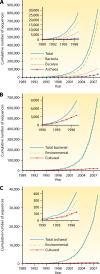
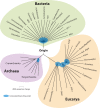
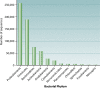
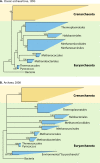
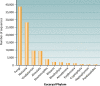
The intent of this article is to provide a critical assessment of our current understanding of life's phylogenetic diversity. Phylogenetic comparison of gene sequences is a natural way to identify microorganisms and can also be used to infer the course of evolution. Three decades of molecular phylogenetic studies with various molecular markers have provided the outlines of a universal tree of life (ToL), the three-domain pattern of archaea, bacteria, and eucarya. The sequence-based perspective on microbial identification additionally opened the way to the identification of environmental microbes without the requirement for culture, particularly through analysis of rRNA gene sequences. Environmental rRNA sequences, which now far outnumber those from cultivars, expand our knowledge of the extent of microbial diversity and contribute increasingly heavily to the emerging ToL. Although the three-domain structure of the ToL is established, the deep phylogenetic structure of each of the domains remains murky and sometimes controversial. Obstacles to accurate inference of deep phylogenetic relationships are both systematic, in molecular phylogenetic calculations, and practical, due to a paucity of sequence representation for many groups of organisms.
Figures
References
-
- Angert, E. R., D. E. Northup, A.-L. Reysenbach, A. S. Peek, B. M. Goebel, and N. R. Pace. 1998. Molecular phylogenetic analysis of a bacterial community in Sulfur River, Parker Cave, Kentucky. Am. Mineralogist 83:1583-1592.
-
- Baldauf, S. L., A. J. Roger, I. Wenk-Siefert, and W. F. Doolittle. 2000. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290:972-977. - PubMed
-
- Bergsten, J. 2005. A review of long-branch attraction. Cladistics 21:163-193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

