Biological diversity of prokaryotic type IV secretion systems
- PMID: 19946141
- PMCID: PMC2786583
- DOI: 10.1128/MMBR.00023-09
Biological diversity of prokaryotic type IV secretion systems
Abstract
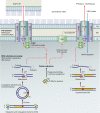
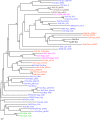
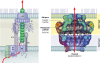
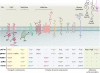
Type IV secretion systems (T4SS) translocate DNA and protein substrates across prokaryotic cell envelopes generally by a mechanism requiring direct contact with a target cell. Three types of T4SS have been described: (i) conjugation systems, operationally defined as machines that translocate DNA substrates intercellularly by a contact-dependent process; (ii) effector translocator systems, functioning to deliver proteins or other macromolecules to eukaryotic target cells; and (iii) DNA release/uptake systems, which translocate DNA to or from the extracellular milieu. Studies of a few paradigmatic systems, notably the conjugation systems of plasmids F, R388, RP4, and pKM101 and the Agrobacterium tumefaciens VirB/VirD4 system, have supplied important insights into the structure, function, and mechanism of action of type IV secretion machines. Information on these systems is updated, with emphasis on recent exciting structural advances. An underappreciated feature of T4SS, most notably of the conjugation subfamily, is that they are widely distributed among many species of gram-negative and -positive bacteria, wall-less bacteria, and the Archaea. Conjugation-mediated lateral gene transfer has shaped the genomes of most if not all prokaryotes over evolutionary time and also contributed in the short term to the dissemination of antibiotic resistance and other virulence traits among medically important pathogens. How have these machines adapted to function across envelopes of distantly related microorganisms? A survey of T4SS functioning in phylogenetically diverse species highlights the biological complexity of these translocation systems and identifies common mechanistic themes as well as novel adaptations for specialized purposes relating to the modulation of the donor-target cell interaction.
Figures




References
-
- Abdallah, A. M., N. C. G. van Pittius, P. A. Champion, J. Cox, J. Luirink, C. M. Vandenbroucke-Grauls, B. J. Appelmelk, and W. Bitter. 2007. Type VII secretion—mycobacteria show the way. Nat. Rev. Microbiol. 5:883-891. - PubMed
-
- Aly, K. A., and C. Baron. 2007. The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 153:3766-3775. - PubMed
-
- Amor, J. C., J. Swails, X. Zhu, C. R. Roy, H. Nagai, A. Ingmundson, X. Cheng, and R. A. Kahn. 2005. The structure of RalF, an ADP-ribosylation factor guanine nucleotide exchange factor from Legionella pneumophila, reveals the presence of a cap over the active site. J. Biol. Chem. 280:1392-1400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

