Dnmt3 and G9a cooperate for tissue-specific development in zebrafish
- PMID: 19946145
- PMCID: PMC2823551
- DOI: 10.1074/jbc.M109.073676
Dnmt3 and G9a cooperate for tissue-specific development in zebrafish
Abstract
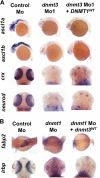
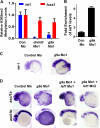
Although DNA methylation is critical for proper embryonic and tissue-specific development, how different DNA methyltransferases affect tissue-specific development and their targets remains unknown. We address this issue in zebrafish through antisense-based morpholino knockdown of Dnmt3 and Dnmt1. Our data reveal that Dnmt3 is required for proper neurogenesis, and its absence results in profound defects in brain and retina. Interestingly, other organs such as intestine remain unaffected suggesting tissue-specific requirements of Dnmt3. Further, comparison of Dnmt1 knockdown phenotypes with those of Dnmt3 suggested that these two families have distinct functions. Consistent with this idea, Dnmt1 failed to complement Dnmt3 deficiency, and Dnmt3 failed to complement Dnmt1 deficiency. Downstream of Dnmt3 we identify a neurogenesis regulator, lef1, as a Dnmt3-specific target gene that is demethylated and up-regulated in dnmt3 morphants. Knockdown of lef1 rescued neurogenesis defects resulting from Dnmt3 absence. Mechanistically, we show cooperation between Dnmt3 and an H3K9 methyltransferase G9a in regulating lef1. Further, like Dnmt1-Suv39h1 cooperativity, Dnmt3 and G9a seemed to function together for tissue-specific development. G9a knockdown, but not Suv39h1 loss, phenocopied dnmt3 morphants and G9a overexpression provided a striking rescue of dnmt3 morphant phenotypes, whereas Suv39h1 overexpression failed, supporting the notion of specific DNMT-histone methyltransferase networks. Consistent with this model, H3K9me3 levels on the lef1 promoter were reduced in both dnmt3 and g9a morphants, and its knockdown rescued neurogenesis defects in g9a morphants. We propose a model wherein specific DNMT-histone methyltransferase networks are utilized to silence critical regulators of cell fate in a tissue-specific manner.
Figures







Similar articles
-
Zebra fish Dnmt1 and Suv39h1 regulate organ-specific terminal differentiation during development.Mol Cell Biol. 2006 Oct;26(19):7077-85. doi: 10.1128/MCB.00312-06. Mol Cell Biol. 2006. PMID: 16980612 Free PMC article.
-
Epigenetic response to environmental stress: Assembly of BRG1-G9a/GLP-DNMT3 repressive chromatin complex on Myh6 promoter in pathologically stressed hearts.Biochim Biophys Acta. 2016 Jul;1863(7 Pt B):1772-81. doi: 10.1016/j.bbamcr.2016.03.002. Epub 2016 Mar 4. Biochim Biophys Acta. 2016. PMID: 26952936 Free PMC article.
-
Direct interaction between DNMT1 and G9a coordinates DNA and histone methylation during replication.Genes Dev. 2006 Nov 15;20(22):3089-103. doi: 10.1101/gad.1463706. Epub 2006 Nov 3. Genes Dev. 2006. PMID: 17085482 Free PMC article.
-
Histone Methyltransferases SUV39H1 and G9a and DNA Methyltransferase DNMT1 in Penumbra Neurons and Astrocytes after Photothrombotic Stroke.Int J Mol Sci. 2021 Nov 19;22(22):12483. doi: 10.3390/ijms222212483. Int J Mol Sci. 2021. PMID: 34830365 Free PMC article.
-
Non-nucleoside inhibitors of DNMT1 and DNMT3 for targeted cancer therapy.Pharmacol Res. 2024 Sep;207:107328. doi: 10.1016/j.phrs.2024.107328. Epub 2024 Jul 28. Pharmacol Res. 2024. PMID: 39079576 Review.
Cited by
-
DNA methyltransferase 3b is dispensable for mouse neural crest development.PLoS One. 2012;7(10):e47794. doi: 10.1371/journal.pone.0047794. Epub 2012 Oct 18. PLoS One. 2012. PMID: 23094090 Free PMC article.
-
Histone 3 lysine 9 trimethylation is differentially associated with isocitrate dehydrogenase mutations in oligodendrogliomas and high-grade astrocytomas.J Neuropathol Exp Neurol. 2013 Apr;72(4):298-306. doi: 10.1097/NEN.0b013e3182898113. J Neuropathol Exp Neurol. 2013. PMID: 23481705 Free PMC article.
-
Epigenetic regulatory mechanisms in vertebrate eye development and disease.Heredity (Edinb). 2010 Jul;105(1):135-51. doi: 10.1038/hdy.2010.16. Epub 2010 Feb 24. Heredity (Edinb). 2010. PMID: 20179734 Free PMC article. Review.
-
The cellular and molecular mechanisms of vertebrate lens development.Development. 2014 Dec;141(23):4432-47. doi: 10.1242/dev.107953. Epub 2014 Nov 18. Development. 2014. PMID: 25406393 Free PMC article. Review.
-
Skeletal Muscle Aging: Lessons From Teleosts.J Gerontol A Biol Sci Med Sci. 2025 May 5;80(6):glae052. doi: 10.1093/gerona/glae052. J Gerontol A Biol Sci Med Sci. 2025. PMID: 38367020 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

