Intervening inhibition underlies simple-cell receptive field structure in visual cortex
- PMID: 19946318
- PMCID: PMC2818750
- DOI: 10.1038/nn.2443
Intervening inhibition underlies simple-cell receptive field structure in visual cortex
Abstract
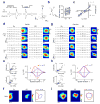
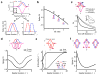
Synaptic inputs underlying spike receptive fields are important for understanding mechanisms of neuronal processing. Using whole-cell voltage-clamp recordings from neurons in mouse primary visual cortex, we examined the spatial patterns of their excitatory and inhibitory synaptic inputs evoked by On and Off stimuli. Neurons with either segregated or overlapped On/Off spike subfields had substantial overlaps between all the four synaptic subfields. The segregated receptive-field structures were generated by the integration of excitation and inhibition with a stereotypic 'overlap-but-mismatched' pattern: the peaks of excitatory On/Off subfields were separated and flanked colocalized peaks of inhibitory On/Off subfields. The small mismatch of excitation/inhibition led to an asymmetric inhibitory shaping of On/Off spatial tunings, resulting in a great enhancement of their distinctiveness. Thus, slightly separated On/Off excitation, together with intervening inhibition, can create simple-cell receptive-field structure and the dichotomy of receptive-field structures may arise from a fine-tuning of the spatial arrangement of synaptic inputs.
Figures


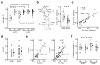

References
-
- Miller KD. Understanding layer 4 of the cortical circuit: a model based on cat V1. Cereb Cortex. 2003;13:73–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

