Inactivation of HAUSP in vivo modulates p53 function
- PMID: 19946331
- PMCID: PMC2857765
- DOI: 10.1038/onc.2009.427
Inactivation of HAUSP in vivo modulates p53 function
Abstract
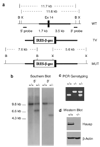
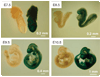
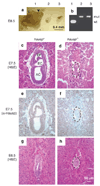
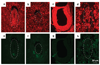
Hausp is a deubiquitinase that has been shown to regulate the p53-Mdm2 pathway. Cotransfection of p53 and Hausp stabilizes p53 through the removal of ubiquitin moieties from polyubiquitinated p53. Interestingly, knockout or RNA interference-mediated knockdown of Hausp in human cells also resulted in the stabilization of p53 due to the destabilization of Mdm2, suggesting a dynamic role of Hausp in p53 activation. To understand the physiological functions of Hausp, we generated hausp knockout mice. Hausp knockout mice die during early embryonic development between embryonic days E6.5 and E7.5. The hausp knockout embryos showed p53 activation, but no apparent increase in apoptosis. Embryonic lethality was caused by a dramatic reduction in proliferation and termination in development, in part due to p53 activation and/or abrogation of p53-independent functions. Although deletion of p53 did not completely rescue the embryonic lethality of the hausp knockout, embryonic development was extended in both hausp and p53 double knockout embryos. These data show that Hausp has a critical role in regulating the p53-Mdm2 pathway.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Amerik AY, Li S, Hochstrasser M. Analysis of the deubiquitinating enzymes of the yeast Saccharomyces cerevisiae. Biol Chem. 2000;381:981–992. - PubMed
-
- Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/ Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–1083. - PubMed
-
- Chen X, Ko LJ, Jayaraman L, Prives C. p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 1996;1:2438–2451. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

