Have last-observation-carried-forward analyses caused us to favour more toxic dementia therapies over less toxic alternatives? A systematic review
- PMID: 19946392
- PMCID: PMC2765769
Have last-observation-carried-forward analyses caused us to favour more toxic dementia therapies over less toxic alternatives? A systematic review
Abstract
Background: Intention-to-treat analysis is used in the analysis of randomized controlled trials to preserve trial power in the presence of missing subject data as well as to control for both known and unknown confounding factors. One form of intention-to-treat analysis is last-observation-carried-forward (LOCF). Concerns exist regarding whether it is appropriate to use LOCF in analyses involving progressive conditions or in situations where missing data are non-random (e.g., subjects drop out because of treatment side effects or differing disease severity).
Objective: To examine the use of intention-to-treat imputation of missing data techniques, and specifically LOCF, in randomized controlled trials of the use of cholinesterase inhibitors and memantine to treat Alzheimer's disease, vascular dementia, mixed dementia and mild cognitive impairment.
Methods: We conducted a systematic electronic search of MEDLINE and the Cochrane Central Register of Controlled Trials from 1984 to 2008 for double-blinded, randomized controlled trials of cholinesterase inhibitors or memantine that examined progressive symptoms in Alzheimer's disease, vascular dementia, mixed dementia and mild cognitive impairment. We collected data on the use of intention-to-treat and non-intention-to-treat analyses and on contraindications to the use of LOCF analysis and we performed quality assessments of included trials.
Results: Of the 57 studies that met the inclusion criteria, 12 did not report intention-to-treat analyses. Of the 34 studies that employed LOCF as the only form of intention-to-treat analysis, 24 reported conditions that could produce biased LOCF analyses favouring the drug under study. The latter finding was more common in cholinesterase inhibitor trials than in memantine studies.
Conclusions: The published results of some randomized controlled trials of dementia drugs may be inaccurate (i.e., drug effectiveness may be exaggerated) or invalid (i.e., there may be false-positive results) because of bias introduced through the inappropriate use of LOCF analyses. This bias favours cholinesterase inhibitors, potentially preventing funding of and patient access to less toxic treatment options such as memantine. Licensing agencies should consider whether to accept LOCF analyses in research on dementias and other chronic progressive conditions.
Conflict of interest statement
Competing interests: None declared.
Figures
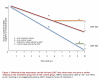


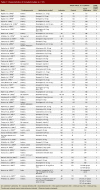



Comment in
-
Missing outcomes in randomized trials: addressing the dilemma.Open Med. 2009 May 12;3(2):e51-3. Open Med. 2009. PMID: 19946393 Free PMC article. No abstract available.
References
-
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366(9503):2112–2117. doi: 10.1016/S0140-6736(05)67889-0. http://linkinghub.elsevier.com/retrieve/pii/S0140673605678890. - DOI - PMC - PubMed
-
- Wimo Anders, Winblad Bengt, Stöffler Albrecht, Wirth Yvonne, Möbius Hans-Jörg. Resource utilisation and cost analysis of memantine in patients with moderate to severe Alzheimer’s disease. Pharmacoeconomics. 2003;21(5):327–340. doi: 10.2165/00019053-200321050-00004. http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=0.... - DOI - PubMed
-
- Unnebrink K, Windeler J. Intention-to-treat: methods for dealing with missing values in clinical trials of progressively deteriorating diseases. Stat Med. 2001;20(24):3931–46. - PubMed
-
- Engels Jean Mundahl, Diehr Paula. Imputation of missing longitudinal data: A comparison of methods. J Clin Epidemiol. 2003;56(10):968–976. doi: 10.1016/S0895-4356(03)00170-7. http://linkinghub.elsevier.com/retrieve/pii/S0895435603001707. - DOI - PubMed
-
- Gadbury G L, Coffey C S, Allison D B. Modern statistical methods for handling missing repeated measurements in obesity trial data: beyond LOCF. Obes Rev. 2003;4(3):175–184. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
