Recent Advances in Anthocyanin Analysis and Characterization
- PMID: 19946465
- PMCID: PMC2783603
- DOI: 10.2174/157341108784587795
Recent Advances in Anthocyanin Analysis and Characterization
Abstract
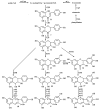
Anthocyanins are a class of polyphenols responsible for the orange, red, purple and blue colors of many fruits, vegetables, grains, flowers and other plants. Consumption of anthocyanins has been linked as protective agents against many chronic diseases and possesses strong antioxidant properties leading to a variety of health benefits. In this review, we examine the advances in the chemical profiling of natural anthocyanins in plant and biological matrices using various chromatographic separations (HPLC and CE) coupled with different detection systems (UV, MS and NMR). An overview of anthocyanin chemistry, prevalence in plants, biosynthesis and metabolism, bioactivities and health properties, sample preparation and phytochemical investigations are discussed while the major focus examines the comparative advantages and disadvantages of each analytical technique.
Figures
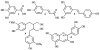
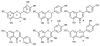





Similar articles
-
Profiling and quantification of grain anthocyanins in purple pericarp × blue aleurone wheat crosses by high-performance thin-layer chromatography and densitometry.Plant Methods. 2018 Mar 31;14:29. doi: 10.1186/s13007-018-0296-5. eCollection 2018. Plant Methods. 2018. PMID: 29610577 Free PMC article.
-
Metabolite profiling of red and blue potatoes revealed cultivar and tissue specific patterns for anthocyanins and other polyphenols.Planta. 2017 Aug;246(2):281-297. doi: 10.1007/s00425-017-2718-4. Epub 2017 Jun 29. Planta. 2017. PMID: 28664422
-
Anthocyanin Pigments: Beyond Aesthetics.Molecules. 2020 Nov 24;25(23):5500. doi: 10.3390/molecules25235500. Molecules. 2020. PMID: 33255297 Free PMC article. Review.
-
Anthocyanins: From Mechanisms of Regulation in Plants to Health Benefits in Foods.Front Plant Sci. 2021 Oct 28;12:748049. doi: 10.3389/fpls.2021.748049. eCollection 2021. Front Plant Sci. 2021. PMID: 34777426 Free PMC article. Review.
-
Plant anthocyanins: Classification, biosynthesis, regulation, bioactivity, and health benefits.Plant Physiol Biochem. 2024 Dec;217:109268. doi: 10.1016/j.plaphy.2024.109268. Epub 2024 Nov 4. Plant Physiol Biochem. 2024. PMID: 39520908 Review.
Cited by
-
Polyphenols: Secondary Metabolites with a Biological Impression.Nutrients. 2024 Aug 3;16(15):2550. doi: 10.3390/nu16152550. Nutrients. 2024. PMID: 39125431 Free PMC article. Review.
-
Extraction of Anthocyanins from Black Grape By-Products and Improving Their Stability Using Cobalt(II) Complexation.Prev Nutr Food Sci. 2022 Dec 31;27(4):457-463. doi: 10.3746/pnf.2022.27.4.457. Prev Nutr Food Sci. 2022. PMID: 36721754 Free PMC article.
-
Strawberry (cv. Romina) Methanolic Extract and Anthocyanin-Enriched Fraction Improve Lipid Profile and Antioxidant Status in HepG2 Cells.Int J Mol Sci. 2017 May 28;18(6):1149. doi: 10.3390/ijms18061149. Int J Mol Sci. 2017. PMID: 28555032 Free PMC article.
-
Effects of anthocyanins on the prevention and treatment of cancer.Br J Pharmacol. 2017 Jun;174(11):1226-1243. doi: 10.1111/bph.13627. Epub 2016 Oct 25. Br J Pharmacol. 2017. PMID: 27646173 Free PMC article. Review.
-
Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice.Int J Mol Sci. 2015 Jan 22;16(2):2352-65. doi: 10.3390/ijms16022352. Int J Mol Sci. 2015. PMID: 25622252 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
