RECENT IMPROVEMENTS IN SMALL ANGLE X-RAY DIFFRACTION FOR THE STUDY OF MUSCLE PHYSIOLOGY
- PMID: 19946470
- PMCID: PMC2783642
- DOI: 10.1088/0034-4885/69/10/R01
RECENT IMPROVEMENTS IN SMALL ANGLE X-RAY DIFFRACTION FOR THE STUDY OF MUSCLE PHYSIOLOGY
Abstract
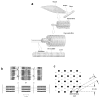
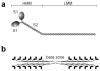
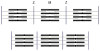
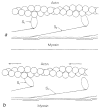
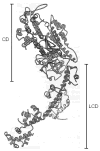
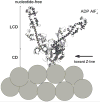
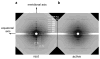
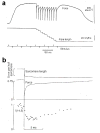
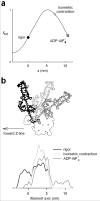
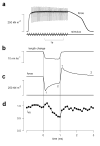
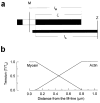
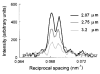
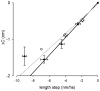
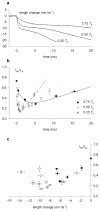
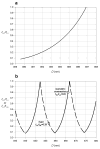
The molecular mechanism of muscle contraction is one of the most important unresolved problems in Biology and Biophysics. Notwithstanding the great advances of recent years, it is not yet known in detail how the molecular motor in muscle, the class II myosin, converts the free energy of ATP hydrolysis into work by interacting with its track, the actin filament, neither it is understood how the high efficiency in energy conversion depends on the cooperative action of myosin motors working in parallel along the actin filament. Researches in muscle contraction imply the combination of mechanical, biochemical and structural methods in studies that span from tissue to single molecule. Therefore, more than for any other research field, progresses in the comprehension of muscle contraction at molecular level are related to, and in turn contribute to, the advancement of methods in Biophysics.This review will focus on the progresses achieved by time resolved small angle X-ray scattering (SAXS) from muscle, an approach made possible by the highly ordered arrangement of both the contractile proteins myosin and actin in the ca 2 mum long structural unit the sarcomere that repeats along the whole length of the muscle cell. Among the time resolved structural techniques, SAXS has proved to be the most powerful method of investigation, as it allows the molecular motor to be studied in situ, in intact single muscle cells, where it is possible to combine the structural study with fast mechanical methods that synchronize the action of the molecular motors. The latest development of this technique allows Angstrom-scale measurements of the axial movement of the motors that pull the actin filament toward the centre of the sarcomere, by exploiting the X-ray interference between the two arrays of myosin motors in the two halves of the sarcomere.
Figures









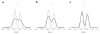

References
-
- Boesecke P, Diat O, Rasmussen B. High brilliance beamline at the European Synchrotron Radiation Facility. Rev Sci Instrum. 1995;66:1636–1638.
-
- Bordas J, Diakun GP, Diaz FG, Harries JE, Lewis RA, Lowy J, Mant GR, Martin-Fernandez ML, Towns-Andrews E. Two-dimensional time-resolved X-ray diffraction studies of live isometrically contracting frog sartorius muscle. J Muscle Res Cell Motil. 1993;14:311–324. - PubMed
-
- Cooke R, Crowder MS, Thomas DD. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982;300:776–778. - PubMed
-
- Cooke R, Franks K. All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry. 1980;19:2265–2269. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
