Progranulin expression is upregulated after spinal contusion in mice
- PMID: 19946692
- PMCID: PMC4290888
- DOI: 10.1007/s00401-009-0616-y
Progranulin expression is upregulated after spinal contusion in mice
Abstract
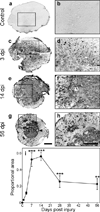
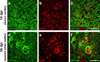
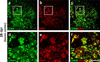
Progranulin (proepithelin) is a pleiotropic growth-factor associated with inflammation and wound repair in peripheral tissues. It also has been implicated in the response to acute traumatic brain injury as well as to chronic neurodegenerative diseases. To determine whether changes in progranulin expression also accompany acute spinal cord injury, C57BL/6 mice were subjected to mid-thoracic (T9 level) contusion spinal cord injury and analyzed by immunohistochemical and biochemical methods. Whereas spinal cord sections prepared from non-injured laminectomy control animals contained low basal levels of progranulin immunoreactivity in gray matter, sections from injured animals contained intense immunoreactivity throughout the injury epicenter that peaked 7-14 days post injury. Progranulin immunoreactivity colocalized with myeloid cell markers CD11b and CD68, indicating that expression increased primarily in activated microglia and macrophages. Immunoblot analysis confirmed that progranulin protein levels rose after injury. On the basis of quantitative polymerase chain reaction analysis, increased protein levels resulted from a tenfold rise in progranulin transcripts. These data demonstrate that progranulin is dramatically induced in myeloid cells after experimental spinal cord injury and is positioned appropriately both spatially and temporally to influence recovery after injury.
Figures


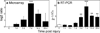
Comment in
-
The enigmatic roles of microglial versus neuronal progranulin in neurological disease.Acta Neuropathol. 2010 Jan;119(1):107-9. doi: 10.1007/s00401-009-0623-z. Epub 2009 Dec 12. Acta Neuropathol. 2010. PMID: 20012633 No abstract available.
Similar articles
-
Exacerbated inflammatory responses related to activated microglia after traumatic brain injury in progranulin-deficient mice.Neuroscience. 2013 Feb 12;231:49-60. doi: 10.1016/j.neuroscience.2012.11.032. Epub 2012 Nov 29. Neuroscience. 2013. PMID: 23201826
-
Progranulin contributes to endogenous mechanisms of pain defense after nerve injury in mice.J Cell Mol Med. 2012 Apr;16(4):708-21. doi: 10.1111/j.1582-4934.2011.01350.x. J Cell Mol Med. 2012. PMID: 21645236 Free PMC article.
-
Progranulin overexpression in sensory neurons attenuates neuropathic pain in mice: Role of autophagy.Neurobiol Dis. 2016 Dec;96:294-311. doi: 10.1016/j.nbd.2016.09.010. Epub 2016 Sep 11. Neurobiol Dis. 2016. PMID: 27629805
-
Cellular effects of progranulin in health and disease.J Mol Neurosci. 2011 Nov;45(3):549-60. doi: 10.1007/s12031-011-9553-z. Epub 2011 May 25. J Mol Neurosci. 2011. PMID: 21611805 Review.
-
The role of PGRN in musculoskeletal development and disease.Front Biosci (Landmark Ed). 2014 Jan 1;19(4):662-71. doi: 10.2741/4234. Front Biosci (Landmark Ed). 2014. PMID: 24389211 Free PMC article. Review.
Cited by
-
A multifaceted role of progranulin in regulating amyloid-beta dynamics and responses.Life Sci Alliance. 2021 Jun 8;4(7):e202000874. doi: 10.26508/lsa.202000874. Print 2021 Jul. Life Sci Alliance. 2021. PMID: 34103390 Free PMC article.
-
Reawakening inflammation in the chronically injured spinal cord using lipopolysaccharide induces diverse microglial states.J Neuroinflammation. 2025 Feb 28;22(1):56. doi: 10.1186/s12974-025-03379-6. J Neuroinflammation. 2025. PMID: 40022205 Free PMC article.
-
Disulfide bonds and disorder in granulin-3: An unusual handshake between structural stability and plasticity.Protein Sci. 2017 Sep;26(9):1759-1772. doi: 10.1002/pro.3212. Epub 2017 Jun 22. Protein Sci. 2017. PMID: 28608407 Free PMC article.
-
CSF-Progranulin and Neurofilament Light Chain Levels in Patients With Radiologically Isolated Syndrome-Sign of Inflammation.Front Neurol. 2018 Dec 18;9:1075. doi: 10.3389/fneur.2018.01075. eCollection 2018. Front Neurol. 2018. PMID: 30619038 Free PMC article.
-
A new immunometabolic perspective of intervertebral disc degeneration.Nat Rev Rheumatol. 2022 Jan;18(1):47-60. doi: 10.1038/s41584-021-00713-z. Epub 2021 Nov 29. Nat Rev Rheumatol. 2022. PMID: 34845360 Review.
References
-
- Baba T, Hoff HB, 3rd, Nemoto H, et al. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol Reprod Dev. 1993;34:233–243. - PubMed
-
- Baker M, Mackenzie IR, Pickering-Brown SM, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

