A facile circular dichroism protocol for rapid determination of enantiomeric excess and concentration of chiral primary amines
- PMID: 19946914
- PMCID: PMC2982703
- DOI: 10.1002/chem.200902650
A facile circular dichroism protocol for rapid determination of enantiomeric excess and concentration of chiral primary amines
Abstract
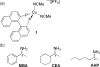
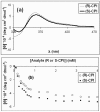
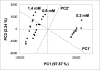
A protocol for the rapid determination of the absolute configuration and enantiomeric excess (ee) of alpha-chiral primary amines with potential applications in asymmetric reaction discovery has been developed. The protocol requires derivatization of alpha-chiral primary amines through condensation with pyridine carboxaldehyde to quantitatively yield the corresponding imine. The Cu(I) complex with 2,2'-bis (diphenylphosphino)-1,1'-dinaphthyl (BINAP--Cu(I)) with the imine yields a metal-to-ligand charge-transfer (MLCT) band in the visible region of the circular dichroism (CD) spectrum upon binding. Diastereomeric host-guest complexes give CD signals of the same signs but different amplitudes, allowing for differentiation of enantiomers. Processing the primary optical data from the CD spectrum with linear discriminant analysis (LDA) allows for the determination of the absolute configuration and identification of the amines, and processing with a supervised multilayer perceptron artificial neural network (MLP-ANN) allows for the simultaneous determination of the ee and concentration. The primary optical data necessary to determine the ee of unknown samples is obtained in two minutes per sample. To demonstrate the utility of the protocol in asymmetric reaction discovery, the ee values and concentrations for an asymmetric metal-catalyzed reaction are determined. The potential of the application of this protocol in high-throughput screening (HTS) of ee is discussed.
Figures

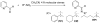
Similar articles
-
Chiral amine enantiomeric excess determination using self-assembled octahedral Fe(II)-imine complexes.Chirality. 2015 Apr;27(4):294-8. doi: 10.1002/chir.22426. Epub 2015 Feb 9. Chirality. 2015. PMID: 25664936 Free PMC article.
-
Rapid optical methods for enantiomeric excess analysis: from enantioselective indicator displacement assays to exciton-coupled circular dichroism.Acc Chem Res. 2014 Jul 15;47(7):2212-21. doi: 10.1021/ar500147x. Epub 2014 Jun 3. Acc Chem Res. 2014. PMID: 24892802
-
In situ assembly of octahedral Fe(II) complexes for the enantiomeric excess determination of chiral amines using circular dichroism spectroscopy.J Am Chem Soc. 2012 Mar 7;134(9):4398-407. doi: 10.1021/ja211768v. Epub 2012 Feb 22. J Am Chem Soc. 2012. PMID: 22272943 Free PMC article.
-
Asymmetric autocatalysis and its application to chiral discrimination.Chirality. 2002 Jul;14(7):548-54. doi: 10.1002/chir.10081. Chirality. 2002. PMID: 12112326 Review.
-
Asymmetric catalysis mediated by the ligand sphere of octahedral chiral-at-metal complexes.Angew Chem Int Ed Engl. 2014 Oct 6;53(41):10868-74. doi: 10.1002/anie.201404305. Epub 2014 Aug 25. Angew Chem Int Ed Engl. 2014. PMID: 25156957 Review.
Cited by
-
Chiral amine enantiomeric excess determination using self-assembled octahedral Fe(II)-imine complexes.Chirality. 2015 Apr;27(4):294-8. doi: 10.1002/chir.22426. Epub 2015 Feb 9. Chirality. 2015. PMID: 25664936 Free PMC article.
-
Optical Activity and Helicity Enhancement of Highly Sensitive Dinaphthylmethane-Based Stereodynamic Probes for Secondary Alcohols.ACS Omega. 2019 Feb 14;4(2):3244-3256. doi: 10.1021/acsomega.8b03337. eCollection 2019 Feb 28. ACS Omega. 2019. PMID: 31459541 Free PMC article.
-
Application of a High-Throughput Enantiomeric Excess Optical Assay Involving a Dynamic Covalent Assembly: Parallel Asymmetric Allylation and Ee Sensing of Homoallylic Alcohols.Chem Sci. 2015 Dec;6(12):6747-6753. doi: 10.1039/C5SC02416A. Epub 2015 Jul 13. Chem Sci. 2015. PMID: 27014433 Free PMC article.
-
An ESI-MS method to determine yield and enantioselectivity in a single assay.J Am Soc Mass Spectrom. 2015 Mar;26(3):397-403. doi: 10.1007/s13361-014-1041-6. Epub 2014 Dec 16. J Am Soc Mass Spectrom. 2015. PMID: 25510928
-
Rapid determination of enantiomeric excess of α-chiral cyclohexanones using circular dichroism spectroscopy.Org Lett. 2011 May 6;13(9):2298-301. doi: 10.1021/ol2004885. Epub 2011 Apr 12. Org Lett. 2011. PMID: 21486023 Free PMC article.
References
-
- Reetz MT. Angew. Chem. 2001;113:292–320. Angew. Chem. Int. Ed. 2001, 40, 284–310.
-
- Welch CJ, Szczerba T, Perrin SR. J. Chromatogr. A. 1997;758:93–98.
- Welch CJ, Grau B, Moore J, Mathre DJ. J. Org. Chem. 2001;66:6836–6837. - PubMed
- Welch CJ, Fleitz F, Antia F, Yehl P, Waters R, Ikemoto N, Armstrong IJD, Mathre D. J. Org. Process Res. Dev. 2004;8:186–191.
- Sigman MS, Jacobsen EN. J. Am. Chem. Soc. 1998;120:4901–4902.
- Wolf PAC, Hawes J. Org. Chem. 2002;67:2727–2729. - PubMed
- Traverse JF, Snapper ML. Drug Discovery Today. 2002;7:1002–1012. - PubMed
- Kuntz KW, Snapper ML, Hoveyda AH. Curr. Opin. Chem. Biol. 1999;3:313–319. - PubMed
-
-
For recently representative examples see: Bailey D M, Hennig A, Uzunova V D, Nau W M. Chem. Eur. J. 2008;14:6069–6077.. Truppo M D, Escalettes F, Turner N J. Angew. Chem. 2008;120:2679–2681. Angew. Chem. Int. Ed. 2008, 47, 2639–2641.. Chen Z-H, He Y-B, Hu C-G, Huang X-H, Hu L. Aust. J.Chem. 2008;61:310–315.. Ingle J R, Busch K W, Kenneth W, Busch M A. Talanta. 2008;75:572–584.. Tanaka H, Matilde S. Chirality. 2008;20:307–312.. Yashima E, Katsuhiro M. Macromolecules. 2008;41:3–12.. Young B L, Cooks R G. Int. J. Mass Spectrom. 2007;267:199–204.. Li Z-B, Lin J, Sabat M, Hyacinth M, Pu L. J. Org, Chem. 2007;72:4905–4916.. Hoogenraad M, Klaus G M, Elders N, Hooijschuur S M, McKay B, Smith A A, Damen EW P. Tetrahedron:Asymmetry. 2004;15:519–523.. van der Linden J B, Ras E-J, Hooijschuur S M, Klaus G M, Luchters N T, Dani P, Verspui G, Smith A A, Damen EW P, McKay B, Hoogenraad M. QSAR Comb. Sci. 2005;24:94–98.. Mazurek S, Ward T R, Novic M. Mol. Divers. 2007;11:141–152.
-
-
-
See for example: Lin J, Zhang H C, Pu L. Org. Lett. 2002;4:3297–3300.. Lee S J, Lin W. J. Am. Chem. Soc. 2002;124:4554–4555.. Ahn K H, Ku H-Y, Kim Y, Kim S-G, Kim Y K, Son H S, Ku J K. Org. Lett. 2003;5:1419–1422.. Pu L. Chem. Rev. 2004;104:1687–1716.. Corradini R, Paganuzzi C, Marchelli R, Pagliari S, Sforza S, Dossena A, Galaverna G, Duchateau A. J. Mater. Chem. 2005;15:2741–2746.. Li Z-B, Lin J, Pu L. Angew. Chem. 2005;117:1718–1721. Angew. Chem. Int. Ed. 2005, 44, 1690–1693.. Zhao J, James T D. J. Mater. Chem. 2005;15:2896–2901.. Mei X, Wolf C. Tetrahedron Lett. 2006;47:7901–7904.. Wolf C, Liu S, Reinhardt B C. Chem. Commun. 2006;47:4242–4244.. Liu S, Pestano J P C, Wolf C. J. Org. Chem. 2008;73:4267–4270.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

