Immune-based therapeutics for pediatric cancer
- PMID: 19947897
- PMCID: PMC2809805
- DOI: 10.1517/14712590903431022
Immune-based therapeutics for pediatric cancer
Abstract
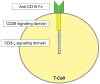
Importance of the field: Although most children with cancer are cured, there remain significant limitations of standard treatment, most notably chemotherapy resistance and non-specific toxicities. Novel immune-based therapies that target pediatric malignancies offer attractive adjuncts and/or alternatives to commonly employed cytotoxic regimens of chemotherapy or radiotherapy. Elucidation of the principles of tumor biology and the development of novel laboratory technologies over the last decade have led to substantial progress in bringing immunotherapies to the bedside.
Areas covered in this review: Current immunotherapeutic clinical trials in pediatric oncology and the science behind their development are reviewed.
What the reader will gain: Most of the immune-based therapies studied to date have been well tolerated, and some have shown promise in the setting of refractory or high-risk malignancies, demonstrating that immunotherapy has the potential to overcome resistance to conventional chemotherapy.
Take home message: Some immune-based therapies, such as ch14.18 and MTP-PE, have already been proven effective in phase III randomized trials. Further studies are needed to optimize and integrate other therapies into standard regimens, and to test them in randomized trials for patients with childhood cancer.
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures

References
-
- Wayne AS, Reaman GH, Helman LJ. Progress in the curative treatment of childhood hematologic malignancies. J Natl Cancer Inst. 2008 Sep 17;100(18):1271–3. - PubMed
-
- Cullen KV, Davey RA, Davey MW. Drug resistance does not correlate with resistance to Fas-mediated apoptosis. Leukemia Research. 2001;25(1):69–75. - PubMed
-
- Giavazzi RR, Bucana CCD, Hart IIR. Correlation of tumor growth inhibitory activity of macrophages exposed to adriamycin and adriamycin sensitivity of the target tumor cells. Journal of the National Cancer Institute. 1984;73(2):447–55. - PubMed
-
- Kontny HU, Lehrnbecher TM, Chanock SJ, Mackall CL. Simultaneous Expression of Fas and Nonfunctional Fas Ligand in Ewing's Sarcoma. Cancer Res. 1998 December 15;58(24):5842–9. 1998. - PubMed
-
- Mapara MY, Sykes M. Tolerance and Cancer: Mechanisms of Tumor Evasion and Strategies for Breaking Tolerance. J Clin Oncol. 2004 March 15;22(6):1136–51. 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
