Proteomic analysis of blastema formation in regenerating axolotl limbs
- PMID: 19948009
- PMCID: PMC2794268
- DOI: 10.1186/1741-7007-7-83
Proteomic analysis of blastema formation in regenerating axolotl limbs
Abstract
Background: Following amputation, urodele salamander limbs reprogram somatic cells to form a blastema that self-organizes into the missing limb parts to restore the structure and function of the limb. To help understand the molecular basis of blastema formation, we used quantitative label-free liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS)-based methods to analyze changes in the proteome that occurred 1, 4 and 7 days post amputation (dpa) through the mid-tibia/fibula of axolotl hind limbs.
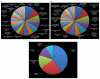
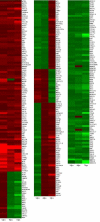
Results: We identified 309 unique proteins with significant fold change relative to controls (0 dpa), representing 10 biological process categories: (1) signaling, (2) Ca2+ binding and translocation, (3) transcription, (4) translation, (5) cytoskeleton, (6) extracellular matrix (ECM), (7) metabolism, (8) cell protection, (9) degradation, and (10) cell cycle. In all, 43 proteins exhibited exceptionally high fold changes. Of these, the ecotropic viral integrative factor 5 (EVI5), a cell cycle-related oncoprotein that prevents cells from entering the mitotic phase of the cell cycle prematurely, was of special interest because its fold change was exceptionally high throughout blastema formation.
Conclusion: Our data were consistent with previous studies indicating the importance of inositol triphosphate and Ca2+ signaling in initiating the ECM and cytoskeletal remodeling characteristic of histolysis and cell dedifferentiation. In addition, the data suggested that blastema formation requires several mechanisms to avoid apoptosis, including reduced metabolism, differential regulation of proapoptotic and antiapoptotic proteins, and initiation of an unfolded protein response (UPR). Since there is virtually no mitosis during blastema formation, we propose that high levels of EVI5 function to arrest dedifferentiated cells somewhere in the G1/S/G2 phases of the cell cycle until they have accumulated under the wound epidermis and enter mitosis in response to neural and epidermal factors. Our findings indicate the general value of quantitative proteomic analysis in understanding the regeneration of complex structures.
Figures



Similar articles
-
Proteomic analysis of fibroblastema formation in regenerating hind limbs of Xenopus laevis froglets and comparison to axolotl.BMC Dev Biol. 2014 Jul 25;14:32. doi: 10.1186/1471-213X-14-32. BMC Dev Biol. 2014. PMID: 25063185 Free PMC article.
-
Regenerative responses in larval axolotl limbs with skin grafts over the amputation surface.J Exp Zool. 1979 Apr;208(1):97-110. doi: 10.1002/jez.1402080111. J Exp Zool. 1979. PMID: 381569
-
Developmental regulation of a matrix metalloproteinase during regeneration of axolotl appendages.Dev Biol. 1994 Dec;166(2):696-703. doi: 10.1006/dbio.1994.1348. Dev Biol. 1994. PMID: 7813787
-
Cell cycle regulation and regeneration.Curr Top Microbiol Immunol. 2013;367:253-76. doi: 10.1007/82_2012_294. Curr Top Microbiol Immunol. 2013. PMID: 23263201 Review.
-
Identification of immune and non-immune cells in regenerating axolotl limbs by single-cell sequencing.Exp Cell Res. 2020 Sep 15;394(2):112149. doi: 10.1016/j.yexcr.2020.112149. Epub 2020 Jun 18. Exp Cell Res. 2020. PMID: 32562784 Free PMC article. Review.
Cited by
-
Comparative transcriptional profiling of the axolotl limb identifies a tripartite regeneration-specific gene program.PLoS One. 2013 May 1;8(5):e61352. doi: 10.1371/journal.pone.0061352. Print 2013. PLoS One. 2013. PMID: 23658691 Free PMC article.
-
Lack of p21 expression links cell cycle control and appendage regeneration in mice.Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5845-50. doi: 10.1073/pnas.1000830107. Epub 2010 Mar 15. Proc Natl Acad Sci U S A. 2010. PMID: 20231440 Free PMC article.
-
The salamander blastema within the broader context of metazoan regeneration.Front Cell Dev Biol. 2023 Aug 11;11:1206157. doi: 10.3389/fcell.2023.1206157. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37635872 Free PMC article. Review.
-
Transcriptomic and proteomic analysis of Hemidactylus frenatus during initial stages of tail regeneration.Sci Rep. 2021 Feb 11;11(1):3675. doi: 10.1038/s41598-021-83283-0. Sci Rep. 2021. PMID: 33574494 Free PMC article.
-
Direct reprogramming of non-limb fibroblasts to cells with properties of limb progenitors.Dev Cell. 2024 Feb 5;59(3):415-430.e8. doi: 10.1016/j.devcel.2023.12.010. Dev Cell. 2024. PMID: 38320485 Free PMC article.
References
-
- Goss RJ. Tissue differentiation in regenerating antlers. Biol Deer Production. 1985;22:229–238.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

