Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism
- PMID: 19948036
- PMCID: PMC2794269
- DOI: 10.1186/1477-7827-7-140
Human male gamete endocrinology: 1alpha, 25-dihydroxyvitamin D3 (1,25(OH)2D3) regulates different aspects of human sperm biology and metabolism
Abstract
Background: A wider biological role of 1alpha,25-Dihydroxyvitamin D3 (1,25(OH)2D3), the active metabolite of vitamin D3, in tissues not primarily related to mineral metabolism was suggested. Recently, we evidenced the ultrastructural localization the 1,25(OH)2D3 receptor in the human sperm. However, the 1,25(OH)2D3 action in human male reproduction has not yet been clarified.
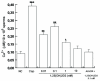
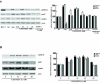
Methods and results: By RT-PCR, Western blot and Immunofluorescence techniques, we demonstrated that human sperm expresses the 1,25(OH)2D3 receptor (VDR). Besides, 25(OH)D3-1 alpha-hydroxylase, evidenced by Western blot analysis, indicated that in sperm 1,25(OH)2D3 is locally produced, highlighting the potential for autocrine-paracrine responses. 1,25(OH)2D3 through VDR, increased intracellular Ca2+ levels, motility and acrosin activity revealing an unexpected significance of this hormone in the acquisition of fertilizing ability. In sperm, 1,25(OH)2D3 through VDR, reduces triglycerides content concomitantly to the increase of lipase activity. Rapid responses stimulated by 1,25(OH)2D3 have been observed on Akt, MAPK and GSK3 implying that this secosteroid is involved in different sperm signalling pathways.
Conclusion: Our data extended the role of 1,25(OH)2D3 beyond its conventional physiological actions, paving the way for novel therapeutic opportunities in the treatment of the male reproduction disorders.
Figures



References
-
- Zehnder D, Bland R, Walker EA, Bradwell AR, Howie AJ, Hewison M, Stewart PM. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in the human kidney. J Am Soc Nephrol. 1999;10:2465–2473. - PubMed
-
- Norman AW, Okamura WH, Hammond MW, Bishop JE, Dormanen MC, Bouillon R, Van Baelen H, Ridall AL, Daane E, Khoury R. Comparison of 6-s-cis and 6-s-trans locked analogs of 1,25(OH)2-vitamin D3 indicates that the 6-s-cis conformation is preferred for rapid nongenomic biological responses and that neither 6-s-cis nor 6-s-trans locked analogs are preferred for genomic biological responses. Mol Endocrinol. 1997;11:1518–1531. doi: 10.1210/me.11.10.1518. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

