Spiperone enhances intracellular calcium level and inhibits the Wnt signaling pathway
- PMID: 19948059
- PMCID: PMC2789054
- DOI: 10.1186/1471-2210-9-13
Spiperone enhances intracellular calcium level and inhibits the Wnt signaling pathway
Abstract
Background: Wnt signaling affects fundamental development pathways by regulating cell proliferation and differentiation. Aberrant activation of Wnt/beta-catenin signaling promotes the development of several cancers and is an attractive target for chemopreventive and chemotherapeutic agents.
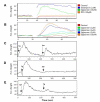
Results: In order to identify the novel antagonists for the Wnt/beta-catenin pathway, we employed a cell-based Wnt reporter system (TOPflash) to screen a library of 960 known drugs. We identified spiperone, a psychotropic drug, as a novel Wnt inhibitor, which specifically blocks canonical Wnt signaling prior to the activation of beta-catenin. The Wnt inhibitory function of spiperone is not associated with its dopamine-, serotonin- and sigma-receptor antagonist properties. Instead, spiperone increases intracellular calcium levels in a similar manner to thapsigargin, that also impedes Wnt signal transduction. Inhibition of protein kinase C had no effect on spiperone-mediated antagonism of Wnt signaling.
Conclusion: Spiperone is a calcium regulator. It inhibits Wnt signaling by enhancing intracellular calcium levels.
Figures


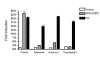

Similar articles
-
Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context.PLoS Biol. 2006 Apr;4(4):e115. doi: 10.1371/journal.pbio.0040115. Epub 2006 Apr 4. PLoS Biol. 2006. PMID: 16602827 Free PMC article.
-
Wnt signaling inhibits cementoblast differentiation and promotes proliferation.Bone. 2009 May;44(5):805-12. doi: 10.1016/j.bone.2008.12.029. Epub 2009 Jan 14. Bone. 2009. PMID: 19442631
-
Differentiation-inducing factor-1 alters canonical Wnt signaling and suppresses alkaline phosphatase expression in osteoblast-like cell lines.J Bone Miner Res. 2006 Aug;21(8):1307-16. doi: 10.1359/jbmr.060512. J Bone Miner Res. 2006. PMID: 16869729
-
Wnt signaling in osteosarcoma.Adv Exp Med Biol. 2014;804:33-45. doi: 10.1007/978-3-319-04843-7_2. Adv Exp Med Biol. 2014. PMID: 24924167 Review.
-
The Wnt/beta-catenin pathway in adrenocortical development and cancer.Mol Cell Endocrinol. 2011 Jan 30;332(1-2):32-7. doi: 10.1016/j.mce.2010.11.014. Epub 2010 Nov 20. Mol Cell Endocrinol. 2011. PMID: 21094679 Review.
Cited by
-
The wnt pathway in mood disorders.Curr Neuropharmacol. 2012 Sep;10(3):239-53. doi: 10.2174/157015912803217279. Curr Neuropharmacol. 2012. PMID: 23449817 Free PMC article.
-
Quercetin Regulates Calcium and Phosphorus Metabolism Through the Wnt Signaling Pathway in Broilers.Front Vet Sci. 2022 Jan 27;8:786519. doi: 10.3389/fvets.2021.786519. eCollection 2021. Front Vet Sci. 2022. PMID: 35155643 Free PMC article.
-
A novel role of protein tyrosine kinase2 in mediating chloride secretion in human airway epithelial cells.PLoS One. 2011;6(7):e21991. doi: 10.1371/journal.pone.0021991. Epub 2011 Jul 13. PLoS One. 2011. PMID: 21765932 Free PMC article.
-
Utility of Extrapolating Human S1500+ Genes to the Whole Transcriptome: Tunicamycin Case Study.Bioinform Biol Insights. 2020 Sep 29;14:1177932220952742. doi: 10.1177/1177932220952742. eCollection 2020. Bioinform Biol Insights. 2020. PMID: 33088175 Free PMC article.
-
The significance of calcium ions in cerebral ischemia-reperfusion injury: mechanisms and intervention strategies.Front Mol Biosci. 2025 May 12;12:1585758. doi: 10.3389/fmolb.2025.1585758. eCollection 2025. Front Mol Biosci. 2025. PMID: 40421420 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

