Reversible, temperature-dependent supramolecular assembly of aquaporin-4 orthogonal arrays in live cell membranes
- PMID: 19948131
- PMCID: PMC2784574
- DOI: 10.1016/j.bpj.2009.09.017
Reversible, temperature-dependent supramolecular assembly of aquaporin-4 orthogonal arrays in live cell membranes
Abstract
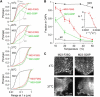
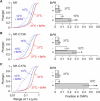
The shorter "M23" isoform of the glial cell water channel aquaporin-4 (AQP4) assembles into orthogonal arrays of particles (OAPs) in cell plasma membranes, whereas the full-length "M1" isoform does not. N-terminal residues are responsible for OAP formation by AQP4-M23 and for blocking of OAP formation in AQP4-M1. In investigating differences in OAP formation by certain N-terminus mutants of AQP4, as measured by freeze-fracture electron microscopy versus live-cell imaging, we discovered reversible, temperature-dependent OAP assembly of certain weakly associating AQP4 mutants. Single-particle tracking of quantum-dot-labeled AQP4 in live cells and total internal reflection fluorescence microscopy showed >80% of M23 in OAPs at 10-50 degrees C compared to <10% of M1. However, OAP formation by N-terminus cysteine-substitution mutants of M1, which probe palmitoylation-regulated OAP assembly, was strongly temperature-dependent, increasing from <10% at 37 degrees C to >70% at 10 degrees C for the double mutant M1-C13A/C17A. OAP assembly by this mutant, but not by native M23, could also be modulated by reducing its membrane density. Exposure of native M1 and single cysteine mutants to 2-bromopalmitate confirmed the presence of regulated OAP assembly by S-palmitoylation. Kinetic studies showed rapid and reversible OAP formation during cooling and OAP disassembly during heating. Our results provide what to our knowledge is the first information on the energetics of AQP4 OAP assembly in plasma membranes.
Figures



References
-
- Hasegawa H., Ma T., Skach W., Matthay M.A., Verkman A.S. Molecular cloning of a mercurial-insensitive water channel expressed in selected water-transporting tissues. J. Biol. Chem. 1994;269:5497–5500. - PubMed
-
- Verkman A.S., Binder D.K., Bloch O., Auguste K., Papadopoulos M.C. Three distinct roles of aquaporin-4 in brain function revealed by knockout mice. Biochim. Biophys. Acta. 2006;1758:1085–1093. - PubMed
-
- Hiroaki Y., Tani K., Kamegawa A., Gyobu N., Nishikawa K. Implications of the aquaporin-4 structure on array formation and cell adhesion. J. Mol. Biol. 2006;355:628–639. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL73856/HL/NHLBI NIH HHS/United States
- R01 EY013574/EY/NEI NIH HHS/United States
- DK86125/DK/NIDDK NIH HHS/United States
- R01 EB000415/EB/NIBIB NIH HHS/United States
- R01 DK035124/DK/NIDDK NIH HHS/United States
- DK72517/DK/NIDDK NIH HHS/United States
- EY13574/EY/NEI NIH HHS/United States
- DK35124/DK/NIDDK NIH HHS/United States
- GM808512/GM/NIGMS NIH HHS/United States
- R01 HL073856/HL/NHLBI NIH HHS/United States
- P30 DK072517/DK/NIDDK NIH HHS/United States
- RC1 DK086125/DK/NIDDK NIH HHS/United States
- EB00415/EB/NIBIB NIH HHS/United States
- R37 DK035124/DK/NIDDK NIH HHS/United States
- R37 EB000415/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources

