An emerging role of mTOR in lipid biosynthesis
- PMID: 19948145
- PMCID: PMC3390254
- DOI: 10.1016/j.cub.2009.09.058
An emerging role of mTOR in lipid biosynthesis
Abstract
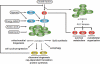
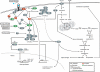
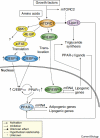
Lipid biosynthesis is essential for the maintenance of cellular homeostasis. The lipids produced by cells (glycerolipids, fatty acids, phospholipids, cholesterol, and sphingolipids) are used as an energy source/reserve, as building blocks for membrane biosynthesis, as precursor molecules for the synthesis of various cellular products, and as signaling molecules. Defects in lipid synthesis or processing contribute to the development of many diseases, including obesity, insulin resistance, type 2 diabetes, non-alcoholic fatty liver disease, and cancer. Studies published over the last few years have shown that the target of rapamycin (TOR), a conserved serine/threonine kinase with an important role in regulating cell growth, controls lipid biosynthesis through various mechanisms. Here, we review these findings and briefly discuss their potential relevance for human health and disease.
Figures
References
-
- Guertin DA, Sabatini DM. Defining the Role of mTOR in Cancer. Cancer cell. 2007;12:9–22. - PubMed
-
- Alessi DR, Pearce LR, Garcia-Martinez JM. New insights into mTOR signaling: mTORC2 and beyond. Sci Signal. 2009;2:e27. - PubMed
-
- Porstmann T, Santos CR, Lewis C, Griffiths B, Schulze A. A new player in the orchestra of cell growth: SREBP activity is regulated by mTORC1 and contributes to the regulation of cell and organ size. Biochem Soc Trans. 2009;37:278–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

