Gliotransmission: Exocytotic release from astrocytes
- PMID: 19948188
- PMCID: PMC2862866
- DOI: 10.1016/j.brainresrev.2009.11.008
Gliotransmission: Exocytotic release from astrocytes
Abstract
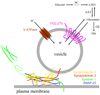
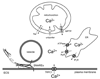
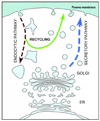
Gliotransmitters are chemicals released from glial cells fulfilling a following set of criteria: (i) they are synthesized by and/or stored in glia; (ii) their regulated release is triggered by physiological and/or pathological stimuli; (iii) they activate rapid (milliseconds to seconds) responses in neighboring cells; and (iv) they play a role in (patho)physiological processes. Astrocytes can release a variety of gliotransmitters into the extracellular space using several different mechanisms. In this review, we focus on exocytotic mechanism(s) underlying the release of three classes of gliotransmitters: (i) amino acids, such as, glutamate and d-serine; (ii) nucleotides, like adenosine 5'-triphosphate; and (iii) peptides, such as, atrial natriuretic peptide and brain-derived neurotrophic factor. It is becoming clear that astrocytes are endowed with elements that qualify them as cells communicating with neurons and other cells within the central nervous system by employing regulated exocytosis.
Copyright 2009 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Regulated exocytosis in astrocytic signal integration.Neurochem Int. 2010 Nov;57(4):451-9. doi: 10.1016/j.neuint.2010.02.007. Epub 2010 Feb 13. Neurochem Int. 2010. PMID: 20156504 Free PMC article. Review.
-
Astroglial excitability and gliotransmission: an appraisal of Ca2+ as a signalling route.ASN Neuro. 2012 Mar 22;4(2):e00080. doi: 10.1042/AN20110061. ASN Neuro. 2012. PMID: 22313347 Free PMC article. Review.
-
Gliotransmission: focus on exocytotic release of L-glutamate and D-serine from astrocytes.Biochem Soc Trans. 2013 Dec;41(6):1557-61. doi: 10.1042/BST20130195. Biochem Soc Trans. 2013. PMID: 24256254 Review.
-
Astrocytic adenosine: from synapses to psychiatric disorders.Philos Trans R Soc Lond B Biol Sci. 2014 Oct 19;369(1654):20130594. doi: 10.1098/rstb.2013.0594. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 25225088 Free PMC article. Review.
-
Astrocytes release ATP/ADP and glutamate in flashes via vesicular exocytosis.Mol Psychiatry. 2025 Jun;30(6):2475-2489. doi: 10.1038/s41380-024-02851-8. Epub 2024 Nov 22. Mol Psychiatry. 2025. PMID: 39578520
Cited by
-
Astrocytic IGF-IRs Induce Adenosine-Mediated Inhibitory Downregulation and Improve Sensory Discrimination.J Neurosci. 2021 Jun 2;41(22):4768-4781. doi: 10.1523/JNEUROSCI.0005-21.2021. Epub 2021 Apr 28. J Neurosci. 2021. PMID: 33911021 Free PMC article.
-
Gq-DREADD Selectively Initiates Glial Glutamate Release and Inhibits Cue-induced Cocaine Seeking.Biol Psychiatry. 2015 Oct 1;78(7):441-51. doi: 10.1016/j.biopsych.2015.02.016. Epub 2015 Feb 24. Biol Psychiatry. 2015. PMID: 25861696 Free PMC article.
-
Function of brain-derived neurotrophic factor in the hypothalamus: Implications for depression pathology.Front Mol Neurosci. 2022 Nov 16;15:1028223. doi: 10.3389/fnmol.2022.1028223. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36466807 Free PMC article. Review.
-
Comparison of unitary exocytic events in pituitary lactotrophs and in astrocytes: modeling the discrete open fusion-pore states.Front Cell Neurosci. 2013 Apr 4;7:33. doi: 10.3389/fncel.2013.00033. eCollection 2013. Front Cell Neurosci. 2013. PMID: 23576951 Free PMC article.
-
Computational quest for understanding the role of astrocyte signaling in synaptic transmission and plasticity.Front Comput Neurosci. 2012 Dec 21;6:98. doi: 10.3389/fncom.2012.00098. eCollection 2012. Front Comput Neurosci. 2012. PMID: 23267326 Free PMC article.
References
-
- Abdipranoto A, Liu GJ, Werry EL, Bennett MR. Mechanisms of secretion of ATP from cortical astrocytes triggered by uridine triphosphate. Neuroreport. 2003;14:2177–2181. - PubMed
-
- Anlauf E, Derouiche A. Astrocytic exocytosis vesicles and glutamate: a high-resolution immunofluorescence study. Glia. 2005;49:96–106. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

