Synthesis and evaluation of 3'-azido-2',3'-dideoxypurine nucleosides as inhibitors of human immunodeficiency virus
- PMID: 19948402
- PMCID: PMC3089953
- DOI: 10.1016/j.bmcl.2009.11.031
Synthesis and evaluation of 3'-azido-2',3'-dideoxypurine nucleosides as inhibitors of human immunodeficiency virus
Abstract
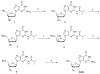
Based on the promising drug resistance profile and potent anti-HIV activity of beta-d-3'-azido-2',3'-dideoxyguanosine, a series of purine modified nucleosides were synthesized by a chemical transglycosylation reaction and evaluated for their antiviral activity, cytotoxicity, and intracellular metabolism. Among the synthesized compounds, several show potent and selective anti-HIV activity in primary lymphocytes.
Published by Elsevier Ltd.
Figures

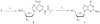


Similar articles
-
Substrate mimicry: HIV-1 reverse transcriptase recognizes 6-modified-3'-azido-2',3'-dideoxyguanosine-5'-triphosphates as adenosine analogs.Nucleic Acids Res. 2012 Jan;40(1):381-90. doi: 10.1093/nar/gkr756. Epub 2011 Sep 13. Nucleic Acids Res. 2012. PMID: 21914723 Free PMC article.
-
Anti-human immunodeficiency virus activity, cross-resistance, cytotoxicity, and intracellular pharmacology of the 3'-azido-2',3'-dideoxypurine nucleosides.Antimicrob Agents Chemother. 2009 Sep;53(9):3715-9. doi: 10.1128/AAC.00392-09. Epub 2009 Jul 13. Antimicrob Agents Chemother. 2009. PMID: 19596885 Free PMC article.
-
Synthesis and antiviral evaluation of unnatural beta-L-enantiomers of 3'-fluoro- and 3'-azido-2',3'-dideoxyguanosine derivatives.Nucleosides Nucleotides Nucleic Acids. 2000 Jan-Feb;19(1-2):205-17. doi: 10.1080/15257770008033004. Nucleosides Nucleotides Nucleic Acids. 2000. PMID: 10772710
-
Structure, Synthesis and Inhibition Mechanism of Nucleoside Analogues as HIV-1 Reverse Transcriptase Inhibitors (NRTIs).ChemMedChem. 2021 Mar 3;16(5):743-766. doi: 10.1002/cmdc.202000695. Epub 2021 Feb 9. ChemMedChem. 2021. PMID: 33230979 Review.
-
Trends in the design of nucleoside analogues as anti-HIV drugs.Curr Pharm Des. 2002;8(8):581-93. doi: 10.2174/1381612024607171. Curr Pharm Des. 2002. PMID: 11945160 Review.
Cited by
-
Synthesis, antiviral activity, cytotoxicity and cellular pharmacology of l-3'-azido-2',3'-dideoxypurine nucleosides.Eur J Med Chem. 2011 Sep;46(9):3832-44. doi: 10.1016/j.ejmech.2011.05.051. Epub 2011 May 30. Eur J Med Chem. 2011. PMID: 21700368 Free PMC article.
-
Binding of novel fullerene inhibitors to HIV-1 protease: insight through molecular dynamics and molecular mechanics Poisson-Boltzmann surface area calculations.J Comput Aided Mol Des. 2011 Oct;25(10):959-76. doi: 10.1007/s10822-011-9475-4. Epub 2011 Oct 4. J Comput Aided Mol Des. 2011. PMID: 21969102
-
Universal profiling of HIV-1 pol for genotypic study and resistance analysis across subtypes.Antivir Ther. 2011;16(8):1267-75. doi: 10.3851/IMP1892. Antivir Ther. 2011. PMID: 22155908 Free PMC article.
-
Synthesis and antiviral evaluation of 2',3'-dideoxy-2',3'-difluoro-D-arabinofuranosyl 2,6-disubstituted purine nucleosides.Heterocycl Comm. 2015;21(5):315-327. doi: 10.1515/hc-2015-0174. Epub 2015 Oct 8. Heterocycl Comm. 2015. PMID: 34316093 Free PMC article.
-
Substrate mimicry: HIV-1 reverse transcriptase recognizes 6-modified-3'-azido-2',3'-dideoxyguanosine-5'-triphosphates as adenosine analogs.Nucleic Acids Res. 2012 Jan;40(1):381-90. doi: 10.1093/nar/gkr756. Epub 2011 Sep 13. Nucleic Acids Res. 2012. PMID: 21914723 Free PMC article.
References
-
- Kaufmann GR, Cooper DA. Curr. Opin. Microbiol. 2000;3:508. - PubMed
-
- Lewis W. Antiviral Ther. 2005;10:M13. - PubMed
- Pillay D, Taylor S, Richman DD. Rev. Med. Virol. 2000;10:231. - PubMed
- Ruane PJ, DeJesus EJ. Acquir. Immun. Defic. Syndr. 2004;37:S21. - PubMed
- Palacios R, Santos J, Camino X, Arazo P, Perea RT, Echevarrfa S, Ribera E, de la Rosa RS, Guillen SM. Curr. Ther. Res. 2005;66:117. - PMC - PubMed
-
-
For example see: Lee K, Choi Y, Gumina G, Zhou W, Schinazi RF, Chu CK. J. Med. Chem. 2002;45:1313.; Schinazi RF, Lloyd RM, Jr., Nguyen MH, Cannon DL, McMillan A, Ilksoy N, Chu CK, Liotta DC, Bazmi HZ, Mellors JW. Antimicrob. Agents Chemother. 1993;37:875.; Feng JY, Shi J, Schinazi RF, Anderson KS. FASEB J. 1999;13:1511.
-
-
- Pathak T. Chem. Rev. 2002;102:1623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

