Heterogeneity in the physiological states and pharmacological responses of differentiating 3T3-L1 preadipocytes
- PMID: 19948481
- PMCID: PMC2779244
- DOI: 10.1083/jcb.200904140
Heterogeneity in the physiological states and pharmacological responses of differentiating 3T3-L1 preadipocytes
Abstract
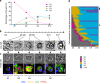
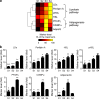
Increases in key components of adipogenesis and lipolysis pathways correlate at the population-averaged level during adipogenesis. However, differentiating preadipocytes are highly heterogeneous in cellular and lipid droplet (LD) morphologies, and the degree to which individual cells follow population-averaged trends is unclear. In this study, we analyze the molecular heterogeneity of differentiating 3T3-L1 preadipocytes using immunofluorescence microscopy. Unexpectedly, we only observe a small percentage of cells with high simultaneous expression of markers for adipogenesis (peroxisome proliferator-activated receptor gamma [PPARgamma], CCAAT/enhancer-binding protein alpha, and adiponectin) and lipid accumulation (hormone-sensitive lipase, perilipin A, and LDs). Instead, we identify subpopulations of cells with negatively correlated expressions of these readouts. Acute perturbation of adipocyte differentiation with PPARgamma agonists, forskolin, and fatty acids induced subpopulation-specific effects, including redistribution of the percentage of cells in observed subpopulations and differential expression levels of PPARgamma. Collectively, our results suggested that heterogeneity observed during 3T3-L1 adipogenesis reflects a dynamic mixture of subpopulations with distinct physiological states.
Figures



References
-
- Arner P., Ostman J. 1978. Relationship between the tissue level of cyclic AMP and the fat cell size of human adipose tissue. J. Lipid Res. 19:613–618 - PubMed
-
- Bauche I.B., El Mkadem S.A., Pottier A.M., Senou M., Many M.C., Rezsohazy R., Penicaud L., Maeda N., Funahashi T., Brichard S.M. 2007. Overexpression of adiponectin targeted to adipose tissue in transgenic mice: impaired adipocyte differentiation. Endocrinology. 148:1539–1549 10.1210/en.2006-0838 - DOI - PubMed

