MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts
- PMID: 19948485
- PMCID: PMC2779245
- DOI: 10.1083/jcb.200904049
MCL-1-dependent leukemia cells are more sensitive to chemotherapy than BCL-2-dependent counterparts
Abstract
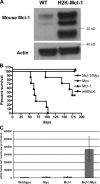
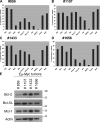
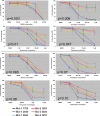
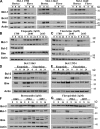
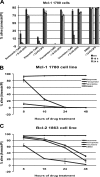
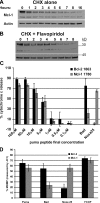
Myeloid cell leukemia sequence 1 (MCL-1) and B cell leukemia/lymphoma 2 (BCL-2) are anti-apoptotic proteins in the BCL-2 protein family often expressed in cancer. To compare the function of MCL-1 and BCL-2 in maintaining cancer survival, we constructed complementary mouse leukemia models based on Emu-Myc expression in which either BCL-2 or MCL-1 are required for leukemia maintenance. We show that the principal anti-apoptotic mechanism of both BCL-2 and MCL-1 in these leukemias is to sequester pro-death BH3-only proteins rather than BAX and BAK. We find that the MCL-1-dependent leukemias are more sensitive to a wide range of chemotherapeutic agents acting by disparate mechanisms. In common across these varied treatments is that MCL-1 protein levels rapidly decrease in a proteosome-dependent fashion, whereas those of BCL-2 are stable. We demonstrate for the first time that two anti-apoptotic proteins can enable tumorigenesis equally well, but nonetheless differ in their influence on chemosensitivity.
Figures




References
-
- Brunelle J.K., Santore M.T., Budinger G.R., Tang Y., Barrett T.A., Zong W.X., Kandel E., Keith B., Simon M.C., Thompson C.B., et al. 2004. c-Myc sensitization to oxygen deprivation-induced cell death is dependent on Bax/Bak, but is independent of p53 and hypoxia-inducible factor-1. J. Biol. Chem. 279:4305–4312 10.1074/jbc.M312241200 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

