Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells
- PMID: 19948499
- PMCID: PMC2779229
- DOI: 10.1083/jcb.200907047
Capacity for stochastic self-renewal and differentiation in mammalian spermatogonial stem cells
Abstract
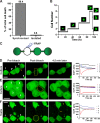
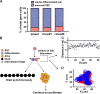
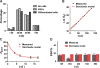
Mammalian spermatogenesis is initiated and sustained by spermatogonial stem cells (SSCs) through self-renewal and differentiation. The basic question of whether SSCs have the potential to specify self-renewal and differentiation in a cell-autonomous manner has yet to be addressed. Here, we show that rat SSCs in ex vivo culture conditions consistently give rise to two distinct types of progeny: new SSCs and differentiating germ cells, even when they have been exposed to virtually identical microenvironments. Quantitative experimental measurements and mathematical modeling indicates that fate decision is stochastic, with constant probability. These results reveal an unexpected ability in a mammalian SSC to specify both self-renewal and differentiation through a self-directed mechanism, and further suggest that this mechanism operates according to stochastic principles. These findings provide an experimental basis for autonomous and stochastic fate choice as an alternative strategy for SSC fate bifurcation, which may also be relevant to other stem cell types.
Figures


Similar articles
-
Conservation of spermatogonial stem cell self-renewal signaling between mouse and rat.Proc Natl Acad Sci U S A. 2005 Oct 4;102(40):14302-7. doi: 10.1073/pnas.0506970102. Epub 2005 Sep 23. Proc Natl Acad Sci U S A. 2005. PMID: 16183739 Free PMC article.
-
Identification of glial cell line-derived neurotrophic factor-regulated genes important for spermatogonial stem cell self-renewal in the rat.Biol Reprod. 2009 Jul;81(1):56-66. doi: 10.1095/biolreprod.108.075358. Epub 2009 Apr 1. Biol Reprod. 2009. PMID: 19339709 Free PMC article.
-
Spermatogonial stem cells derived from infertile Wv/Wv mice self-renew in vitro and generate progeny following transplantation.Biol Reprod. 2009 Aug;81(2):293-301. doi: 10.1095/biolreprod.109.075960. Epub 2009 Apr 15. Biol Reprod. 2009. PMID: 19369648 Free PMC article.
-
The identity and fate decision control of spermatogonial stem cells: where is the point of no return?Curr Top Dev Biol. 2013;102:61-95. doi: 10.1016/B978-0-12-416024-8.00003-9. Curr Top Dev Biol. 2013. PMID: 23287030 Review.
-
[Biological properties of spermatogonial stem cell niches].Zhonghua Nan Ke Xue. 2012 Apr;18(4):359-63. Zhonghua Nan Ke Xue. 2012. PMID: 22574376 Review. Chinese.
Cited by
-
Developmental lineage priming in Dictyostelium by heterogeneous Ras activation.Elife. 2013 Nov 26;2:e01067. doi: 10.7554/eLife.01067. Elife. 2013. PMID: 24282234 Free PMC article.
-
Undifferentiated primate spermatogonia and their endocrine control.Trends Endocrinol Metab. 2010 Aug;21(8):488-95. doi: 10.1016/j.tem.2010.03.001. Epub 2010 Mar 30. Trends Endocrinol Metab. 2010. PMID: 20359909 Free PMC article.
-
Development of quantitative microscopy-based assays for evaluating dynamics of living cultures of mouse spermatogonial stem/progenitor cells.Biol Reprod. 2012 Oct 18;87(4):90. doi: 10.1095/biolreprod.112.101717. Print 2012 Oct. Biol Reprod. 2012. PMID: 22933516 Free PMC article.
-
Regulation of mouse spermatogonial stem cell differentiation by STAT3 signaling.Biol Reprod. 2010 Sep;83(3):427-33. doi: 10.1095/biolreprod.109.083352. Epub 2010 May 26. Biol Reprod. 2010. PMID: 20505165 Free PMC article.
-
The in vivo response of stem and other undifferentiated spermatogonia to the reversible inhibition of glial cell line-derived neurotrophic factor signaling in the adult.Stem Cells. 2012 Apr;30(4):732-40. doi: 10.1002/stem.1028. Stem Cells. 2012. PMID: 22232066 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

