Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon
- PMID: 19948652
- PMCID: PMC2825607
- DOI: 10.1113/jphysiol.2009.181172
Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon
Abstract
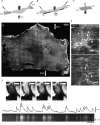
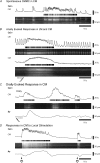
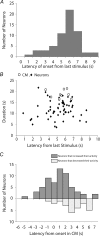
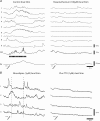
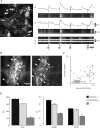
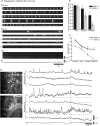
The spontaneous colonic migrating motor complex (CMMC) is a cyclical contractile and electrical event that is the primary motor pattern underlying fecal pellet propulsion along the murine colon. We have combined Ca(2+) imaging with immunohistochemistry to determine the role of different classes of myenteric neurons during the CMMC. Between CMMCs, myenteric neurons usually displayed ongoing but uncoordinated activity. Stroking the mucosa at the oral or anal end of the colon resulted in a CMMC (latency: 6 to 10 s; duration: 28 s) that consisted of prolonged increases in activity in many myenteric neurons that was correlated to Ca(2+) transients in and displacement of the muscle. These neurons were likely excitatory motor neurons. Activity in individual neurons during the CMMC was similar regardless of whether the CMMC occurred spontaneously or was evoked by anal or oral mucosal stimulation. This suggests that convergent interneuronal pathways exist which generate CMMCs. Interestingly, Ca(2+) transients in a subset of NOS +ve neurons were substantially reduced during the CMMC. These neurons are likely to be inhibitory motor neurons that reduce their activity during a complex (disinhibition) to allow full excitation of the muscle. Local stimulation of the mucosa evoked synchronized Ca(2+) transients in Dogiel Type II (mitotracker/calbindin-positive) neurons after a short delay (1-2 s), indicating they were the sensory neurons underlying the CMMC. These local responses were observed in hexamethonium, but were blocked by ondansetron (5-HT(3) antagonist), suggesting Dogiel Type II neurons were activated by 5-HT release from enterochromaffin cells in the mucosa. In fact, removal of the mucosa yielded no spontaneous CMMCs, although many neurons (NOS +ve and NOS ve) exhibited ongoing activity, including Dogiel Type II neurons. These results suggest that spontaneous or evoked 5-HT release from the mucosa is necessary for the activation of Dogiel Type II neurons that generate CMMCs.
Figures




References
-
- Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience. 2000;101:459–469. - PubMed
-
- Bush TG, Spencer NJ, Watters N, Sanders KM, Smith TK. Spontaneous migrating motor complexes occur in both the terminal ileum and colon of the C57BL/6 mouse in vitro. Auton Neurosci. 2000;84:162–168. - PubMed
-
- Christensen J, Anuras S, Arthur C. Influence of intrinsic nerves on electromyogram of cat colon in vitro. Am J Physiol Endocrinol Metab. 1978;234:E641–647. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

