Modulation of the cold-activated cation channel TRPM8 by surface charge screening
- PMID: 19948654
- PMCID: PMC2821726
- DOI: 10.1113/jphysiol.2009.183582
Modulation of the cold-activated cation channel TRPM8 by surface charge screening
Abstract
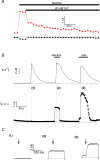
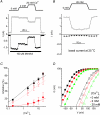
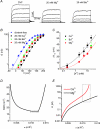
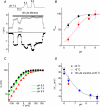
TRPM8, a cation channel activated by cold and by cooling agents such as menthol and icilin, is critically involved in somatosensory cold sensation. Ion fluxes through TRPM8 are highly sensitive to changes in extracellular Ca(2+) and pH, but the mechanisms underlying this type of modulation are poorly understood. Here we provide evidence that inhibition of TRPM8 currents by extracellular divalent cations and protons is due to surface charge screening. We demonstrate that increasing concentrations of divalent cations or protons cause parallel shifts of the voltage dependence of TRPM8 activation towards positive potentials. These shifts were interpreted using the Gouy-Chapman-Stern theory, yielding an estimate for the density of fixed negative surface charge between 0.0098 and 0.0126 equivalent charges per A(2). These results represent the first description of the effects of surface charge screening on a TRP channel and provide a straightforward explanation for the known effects of extracellular Ca(2+) on cold-sensitive neurons.
Figures
References
-
- Agarwal A, Dhiraj S, Raza M, Pandey R, Pandey CK, Singh PK, Singh U, Gupta D. Vein pretreatment with magnesium sulfate to prevent pain on injection of propofol is not justified. Can J Anaesth. 2004;51:130–133. - PubMed
-
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

