Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles
- PMID: 19948655
- PMCID: PMC2821527
- DOI: 10.1113/jphysiol.2009.181008
Two novel/ancient myosins in mammalian skeletal muscles: MYH14/7b and MYH15 are expressed in extraocular muscles and muscle spindles
Abstract
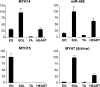
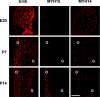
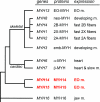
The mammalian genome contains three ancient sarcomeric myosin heavy chain (MYH) genes, MYH14/7b, MYH15 and MYH16, in addition to the two well characterized clusters of skeletal and cardiac MYHs. MYH16 is expressed in jaw muscles of carnivores; however the expression pattern of MYH14 and MYH15 is not known. MYH14 and MYH15 orthologues are present in frogs and birds, coding for chicken slow myosin 2 and ventricular MYH, respectively, whereas only MYH14 orthologues have been detected in fish. In all species the MYH14 gene contains a microRNA, miR-499. Here we report that in rat and mouse, MYH14 and miR-499 transcripts are detected in heart, slow muscles and extraocular (EO) muscles, whereas MYH15 transcripts are detected exclusively in EO muscles. However, MYH14 protein is detected only in a minor fibre population in EO muscles, corresponding to slow-tonic fibres, and in bag fibres of muscle spindles. MYH15 protein is present in most fibres of the orbital layer of EO muscles and in the extracapsular region of bag fibres. During development, MYH14 is expressed at low levels in skeletal muscles, heart and all EO muscle fibres but disappears from most fibres, except the slow-tonic fibres, after birth. In contrast, MYH15 is absent in embryonic and fetal muscles and is first detected after birth in the orbital layer of EO muscles. The identification of the expression pattern of MYH14 and MYH15 brings to completion the inventory of the MYH isoforms involved in sarcomeric architecture of skeletal muscles and provides an unambiguous molecular basis to study the contractile properties of slow-tonic fibres in mammals.
Figures



Similar articles
-
Expression and identification of 10 sarcomeric MyHC isoforms in human skeletal muscles of different embryological origin. Diversity and similarity in mammalian species.Ann Anat. 2016 Sep;207:9-20. doi: 10.1016/j.aanat.2016.02.007. Epub 2016 Mar 9. Ann Anat. 2016. PMID: 26970499
-
The ancient sarcomeric myosins found in specialized muscles.Skelet Muscle. 2019 Mar 5;9(1):7. doi: 10.1186/s13395-019-0192-3. Skelet Muscle. 2019. PMID: 30836986 Free PMC article. Review.
-
Molecular cloning and mRNA expression analysis of carp embryonic, slow and cardiac myosin heavy chain isoforms.J Exp Biol. 2006 Jan;209(Pt 1):188-98. doi: 10.1242/jeb.01978. J Exp Biol. 2006. PMID: 16354789
-
Spatial and temporal patterns of myosin heavy chain expression in developing rat extraocular muscle.J Muscle Res Cell Motil. 1996 Jun;17(3):297-312. doi: 10.1007/BF00240928. J Muscle Res Cell Motil. 1996. PMID: 8814550
-
The mammalian myosin heavy chain gene family.Annu Rev Cell Dev Biol. 1996;12:417-39. doi: 10.1146/annurev.cellbio.12.1.417. Annu Rev Cell Dev Biol. 1996. PMID: 8970733 Review.
Cited by
-
Remarkable heterogeneity in myosin heavy-chain composition of the human young masseter compared with young biceps brachii.Histochem Cell Biol. 2012 Oct;138(4):669-82. doi: 10.1007/s00418-012-0985-5. Epub 2012 Jul 10. Histochem Cell Biol. 2012. PMID: 22777345
-
Myosin heavy chain 15 is associated with bovine pulmonary arterial pressure.Pulm Circ. 2014 Sep;4(3):496-503. doi: 10.1086/677364. Pulm Circ. 2014. PMID: 25621163 Free PMC article.
-
RNA-seq reveals transcriptome changes in goats following myostatin gene knockout.PLoS One. 2017 Dec 11;12(12):e0187966. doi: 10.1371/journal.pone.0187966. eCollection 2017. PLoS One. 2017. PMID: 29228005 Free PMC article.
-
Motor neuron vulnerability and resistance in amyotrophic lateral sclerosis.Acta Neuropathol. 2017 Jun;133(6):863-885. doi: 10.1007/s00401-017-1708-8. Epub 2017 Apr 13. Acta Neuropathol. 2017. PMID: 28409282 Free PMC article. Review.
-
Circulating miR-499a-5p Is a Potential Biomarker of MYH7-Associated Hypertrophic Cardiomyopathy.Int J Mol Sci. 2022 Mar 30;23(7):3791. doi: 10.3390/ijms23073791. Int J Mol Sci. 2022. PMID: 35409153 Free PMC article.
References
-
- Bisaha JG, Bader D. Identification and characterization of a ventricular-specific avian myosin heavy chain, VMHC1: expression in differentiating cardiac and skeletal muscle. Dev Biol. 1991;148:355–364. - PubMed
-
- Bormioli SP, Torresan P, Sartore S, Moschini GB, Schiaffino S. Immunohistochemical identification of slow-tonic fibres in human extrinsic eye muscles. Invest Ophthalmol Vis Sci. 1979;18:303–306. - PubMed
-
- Brueckner JK, Porter JD. Visual system maldevelopment disrupts extraocular muscle-specific myosin expression. J Appl Physiol. 1998;85:584–592. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

