The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells
- PMID: 19948717
- PMCID: PMC2823438
- DOI: 10.1074/jbc.M109.038398
The linker for activation of B cells (LAB)/non-T cell activation linker (NTAL) regulates triggering receptor expressed on myeloid cells (TREM)-2 signaling and macrophage inflammatory responses independently of the linker for activation of T cells
Abstract
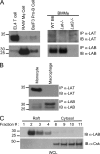
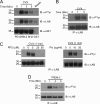
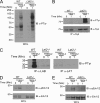
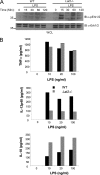
Triggering receptor expressed on myeloid cells-2 (TREM-2) is rapidly emerging as a key regulator of the innate immune response via its regulation of macrophage inflammatory responses. Here we demonstrate that proximal TREM-2 signaling parallels other DAP12-based receptor systems in its use of Syk and Src-family kinases. However, we find that the linker for activation of T cells (LAT) is severely reduced as monocytes differentiate into macrophages and that TREM-2 exclusively uses the linker for activation of B cells (LAB encoded by the gene Lat2(-/-)) to mediate downstream signaling. LAB is required for TREM-2-mediated activation of Erk1/2 and dampens proximal TREM-2 signals through a novel LAT-independent mechanism resulting in macrophages with proinflammatory properties. Thus, Lat2(-/-) macrophages have increased TREM-2-induced proximal phosphorylation, and lipopolysaccharide stimulation of these cells leads to increased interleukin-10 (IL-10) and decreased IL-12p40 production relative to wild type cells. Together these data identify LAB as a critical, LAT-independent regulator of TREM-2 signaling and macrophage development capable of controlling subsequent inflammatory responses.
Figures
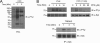
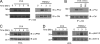


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

