Inducible nephrin transgene expression in podocytes rescues nephrin-deficient mice from perinatal death
- PMID: 19948823
- PMCID: PMC2797869
- DOI: 10.2353/ajpath.2010.080843
Inducible nephrin transgene expression in podocytes rescues nephrin-deficient mice from perinatal death
Abstract
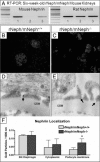
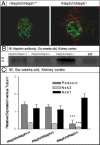
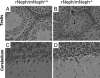
Mutations leading to nephrin loss result in massive proteinuria both in humans and mice. Early perinatal lethality of conventional nephrin knockout mice makes it impossible to determine the role of nephrin protein in the adult kidney and in extra-renal tissues. Herein, we studied whether podocyte-specific, doxycycline-inducible, rat nephrin expression can rescue nephrin-deficient mice from perinatal lethality. Fourteen littermates out of 72 lacked endogenous nephrin and expressed transgenic rat nephrin. Six of these rescued mice survived until 6 weeks of age, whereas the nephrin-deficient pups died before the age of 5 days. The rescued mice were smaller, developed proteinuria, and showed histological abnormalities in the kidney. Despite foot process effacement, slit diaphragms were observed. Importantly, the expression and localization of several proteins associated with the signaling capacity of nephrin or the regulation of the expression of nephrin were changed in the podocytes. Indeed, all rescued mice showed impaired locomotor activity and distinct histological abnormalities in the cerebellum, and the male mice were also infertile and showed genital malformations. These observations are consistent with normal nephrin expression in the testis and cerebellum. These observations indicate that podocyte-specific expression of rat nephrin can rescue nephrin-deficient mice from perinatal death, but is not sufficient for full complementation.
Figures








References
-
- Rapola J. Congenital nephrotic syndrome. Pediatr Nephrol. 1987;1:441–446. - PubMed
-
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. - PubMed
-
- Lenkkeri U, Mannikko M, McCready P, Lamerdin J, Gribouval O, Niaudet PM, Antignac CK, Kashtan CE, Homberg C, Olsen A, Kestila M, Tryggvason K. Structure of the gene for congenital nephrotic syndrome of the finnish type (NPHS1) and characterization of mutations. Am J Hum Genet. 1999;64:51–61. - PMC - PubMed
-
- Hallman N, Hjelt L. Congenital nephrotic syndrome. J Pediatr. 1959;55:152–162. - PubMed
-
- Tryggvason K, Kouvalainen K. Number of nephrons in normal human kidneys and kidneys of patients with the congenital nephrotic syndrome. A study using a sieving method for counting of glomeruli. Nephron. 1975;15:62–68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

