Methyl donor deficiency affects fetal programming of gastric ghrelin cell organization and function in the rat
- PMID: 19948829
- PMCID: PMC2797889
- DOI: 10.2353/ajpath.2010.090153
Methyl donor deficiency affects fetal programming of gastric ghrelin cell organization and function in the rat
Abstract
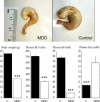
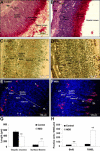
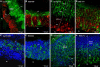
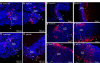
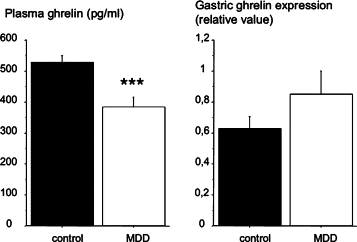
Methyl donor deficiency (MDD) during pregnancy influences intrauterine development. Ghrelin is expressed in the stomach of fetuses and influences fetal growth, but MDD influence on gastric ghrelin is unknown. We examined the gastric ghrelin system in MDD-induced intrauterine growth retardation. By using specific markers and approaches (such as periodic acid-Schiff, bromodeoxyuridine, homocysteine, terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling, immunostaining, reverse transcription-polymerase chain reaction), we studied the gastric oxyntic mucosa cellular organization and ghrelin gene expression in the mucosa in 20-day-old fetuses and weanling pups, and plasma ghrelin concentration in weanling rat pups of dams either normally fed or deprived of choline, folate, vitamin B6, and vitamin B12 during gestation and suckling periods. MDD fetuses weighed less than controls; the weight deficit reached 57% at weaning (P < 0.001). Both at the end of gestation and at weaning, they presented with an aberrant gastric oxyntic mucosa formation with loss of cell polarity, anarchic cell migration, abnormal progenitor differentiation, apoptosis, and signs of surface layer erosion. Ghrelin cells were abnormally located in the pit region of oxyntic glands. At weaning, plasma ghrelin levels were decreased (-28%; P < 0.001) despite unchanged mRNA expression in the stomach. This decrease was associated with lower body weight. Taken together, these data indicate that one mechanism through which MDD influences fetal programming is the remodeling of gastric cellular organization, leading to dysfunction of the ghrelin system and dramatic effects on growth.
Figures
Similar articles
-
Ghrelin and GHS-R in the rat gastric mucosa: Are they involved in regulation of growth during early weaning?Nutrition. 2016 Jan;32(1):101-7. doi: 10.1016/j.nut.2015.06.014. Epub 2015 Jul 26. Nutrition. 2016. PMID: 26520918
-
Methyl donor deficiency affects small-intestinal differentiation and barrier function in rats.Br J Nutr. 2013 Feb 28;109(4):667-77. doi: 10.1017/S0007114512001869. Epub 2012 Jul 16. Br J Nutr. 2013. PMID: 22794784
-
Early methyl donor deficiency produces severe gastritis in mothers and offspring through N-homocysteinylation of cytoskeleton proteins, cellular stress, and inflammation.FASEB J. 2013 Jun;27(6):2185-97. doi: 10.1096/fj.12-224642. Epub 2013 Feb 11. FASEB J. 2013. PMID: 23401564
-
Ghrelin and Helicobacter pylori infection.World J Gastroenterol. 2008 Nov 7;14(41):6327-33. doi: 10.3748/wjg.14.6327. World J Gastroenterol. 2008. PMID: 19009647 Free PMC article. Review.
-
Ghrelin and the growth hormone secretagogue receptor in growth and development.Int J Obes (Lond). 2009 Apr;33 Suppl 1:S48-52. doi: 10.1038/ijo.2009.17. Int J Obes (Lond). 2009. PMID: 19363508 Review.
Cited by
-
Late Maternal Folate Supplementation Rescues from Methyl Donor Deficiency-Associated Brain Defects by Restoring Let-7 and miR-34 Pathways.Mol Neurobiol. 2017 Sep;54(7):5017-5033. doi: 10.1007/s12035-016-0035-8. Epub 2016 Aug 17. Mol Neurobiol. 2017. PMID: 27534418 Free PMC article.
-
Methyl donor-deficient diet during development can affect fear and anxiety in adulthood in C57BL/6J mice.PLoS One. 2014 Aug 21;9(8):e105750. doi: 10.1371/journal.pone.0105750. eCollection 2014. PLoS One. 2014. PMID: 25144567 Free PMC article.
-
Methyl-donor supplementation prevents intestinal colonization by Adherent-Invasive E. coli in a mouse model of Crohn's disease.Sci Rep. 2020 Jul 31;10(1):12922. doi: 10.1038/s41598-020-69472-3. Sci Rep. 2020. PMID: 32737335 Free PMC article.
-
Effects of maternal methyl donor intake during pregnancy on ileum methylation and function in an intrauterine growth restriction pig model.J Anim Sci Biotechnol. 2024 Feb 4;15(1):19. doi: 10.1186/s40104-023-00970-w. J Anim Sci Biotechnol. 2024. PMID: 38310243 Free PMC article.
-
Methyl deficient diet aggravates experimental colitis in rats.J Cell Mol Med. 2011 Nov;15(11):2486-97. doi: 10.1111/j.1582-4934.2010.01252.x. J Cell Mol Med. 2011. PMID: 21199330 Free PMC article.
References
-
- Lindenbaum J, Rosenberg I, Wilson P, Stabler S, Allen R. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. - PubMed
-
- Herrmann W, Schorr H, Purschwitz K, Rassoul F, Richter V. Total homocysteine, vitamin B(12), and total antioxidant status in vegetarians. Clin Chem. 2001;47:1094–1101. - PubMed
-
- Stabler S, Allen R. Vitamin B12 deficiency as a worldwide problem. Annu Rev Nutr. 2004;24:299–326. - PubMed
-
- Finkelstein J. The metabolism of homocysteine: pathways and regulation. Eur J Pediatr. 1998;157:S40–S44. - PubMed
-
- Mattson M, Shea T. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003;26:137–146. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

