CD11c+ cells are required to prevent progression from local acute lung injury to multiple organ failure and death
- PMID: 19948830
- PMCID: PMC2797884
- DOI: 10.2353/ajpath.2010.081027
CD11c+ cells are required to prevent progression from local acute lung injury to multiple organ failure and death
Abstract
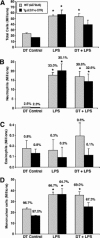
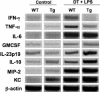
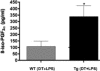
To investigate the role of CD11c(+) cells in endotoxin-induced acute lung injury, wild-type or CD11c-diphtheria toxin receptor transgenic mice were treated with intraperitoneal diphtheria toxin (5 ng/g b.wt.) in the presence or absence of intratracheal lipopolysaccharide (51 microg). Lipopolysaccharide treatment resulted in 100% mortality in CD11c-depleted animals but not in control animals. Analysis of local lung tissue revealed no differences in acute lung injury severity; however, analysis of distal tissues revealed severe damage and necrosis to multiple organs (liver, spleen, and kidneys) in CD11c-diphtheria toxin receptor mice but not in wild-type mice. In addition, dramatic increases in systemic levels of liver enzymes (alanine aminotransferase, 657 U/L, aspartate aminotransferase, 1401 U/L), blood urea (53 mg/dl), and 8-iso-prostaglandin F(2alpha), a marker of oxidative stress (350 pg/ml), were observed. These data demonstrate that CD11c(+) cells play a critical role in protecting the organs from systemic injury caused by a pulmonary endotoxin challenge.
Figures


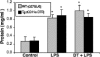
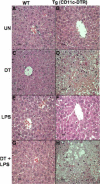
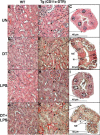
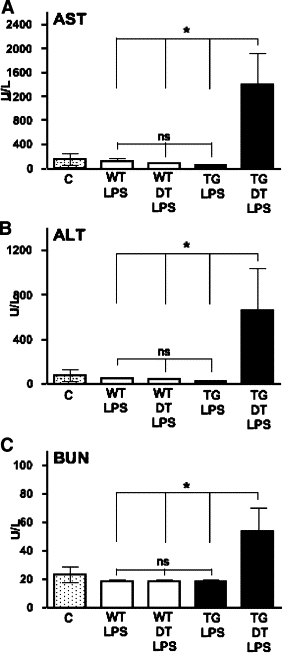
References
-
- Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. - PubMed
-
- Ware LB. Pathophysiology of acute lung injury and the acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:337–349. - PubMed
-
- Frutos-Vivar F, Ferguson ND, Esteban A. Epidemiology of acute lung injury and acute respiratory distress syndrome. Semin Respir Crit Care Med. 2006;27:327–336. - PubMed
-
- Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202:145–156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

