The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms
- PMID: 19948837
- PMCID: PMC2812187
- DOI: 10.1128/IAI.00881-09
The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms
Abstract
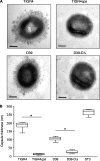
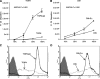
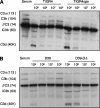
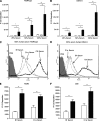
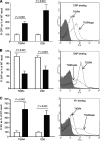
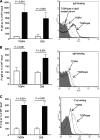
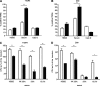
The Streptococcus pneumoniae capsule is vital for virulence and may inhibit complement activity and phagocytosis. However, there are only limited data on the mechanisms by which the capsule affects complement and the consequences for S. pneumoniae interactions with phagocytes. Using unencapsulated serotype 2 and 4 S. pneumoniae mutants, we have confirmed that the capsule has several effects on complement activity. The capsule impaired bacterial opsonization with C3b/iC3b by both the alternative and classical complement pathways and also inhibited conversion of C3b bound to the bacterial surface to iC3b. There was increased binding of the classical pathway mediators immunoglobulin G (IgG) and C-reactive protein (CRP) to unencapsulated S. pneumoniae, indicating that the capsule could inhibit classical pathway complement activity by masking antibody recognition of subcapsular antigens, as well as by inhibiting CRP binding. Cleavage of serum IgG by the enzyme IdeS reduced C3b/iC3b deposition on all of the strains, but there were still marked increases in C3b/iC3b deposition on unencapsulated TIGR4 and D39 strains compared to encapsulated strains, suggesting that the capsule inhibits both IgG-mediated and IgG-independent complement activity against S. pneumoniae. Unencapsulated strains were more susceptible to neutrophil phagocytosis after incubation in normal serum, normal serum treated with IdeS, complement-deficient serum, and complement-deficient serum treated with IdeS or in buffer alone, suggesting that the capsule inhibits phagocytosis mediated by Fcgamma receptors, complement receptors, and nonopsonic receptors. Overall, these data show that the S. pneumoniae capsule affects multiple aspects of complement- and neutrophil-mediated immunity, resulting in a profound inhibition of opsonophagocytosis.
Figures
References
-
- Bentley, S. D., D. M. Aanensen, A. Mavroidi, D. Saunders, E. Rabbinowitsch, M. Collins, K. Donohoe, D. Harris, L. Murphy, M. A. Quail, G. Samuel, I. C. Skovsted, M. S. Kaltoft, B. Barrell, P. R. Reeves, J. Parkhill, and B. G. Spratt. 2006. Genetic analysis of the capsular biosynthetic locus from all 90 pneumococcal serotypes. PLoS Genet. 2:e31. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

