Effect of biostimulation and bioaugmentation on degradation of polyurethane buried in soil
- PMID: 19948849
- PMCID: PMC2813001
- DOI: 10.1128/AEM.00534-09
Effect of biostimulation and bioaugmentation on degradation of polyurethane buried in soil
Abstract
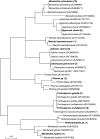
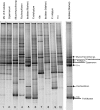
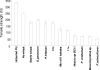
This work investigated biostimulation and bioaugmentation as strategies for removing polyurethane (PU) waste in soil. Soil microcosms were biostimulated with the PU dispersion agent "Impranil" and/or yeast extract or were bioaugmented with PU-degrading fungi, and the degradation of subsequently buried PU was determined. Fungal communities in the soil and colonizing buried PU were enumerated on solid media and were analyzed using denaturing gradient gel electrophoresis (DGGE). Biostimulation with yeast extract alone or in conjunction with Impranil increased PU degradation 62% compared to the degradation in untreated control soil and was associated with a 45% increase in putative PU degraders colonizing PU. Specific fungi were enriched in soil following biostimulation; however, few of these fungi colonized the surface of buried PU. Fungi used for soil bioaugmentation were cultivated on the surface of sterile wheat to form a mycelium-rich inoculum. Wheat, when added alone to soil, increased PU degradation by 28%, suggesting that wheat biomass had a biostimulating effect. Addition of wheat colonized with Nectria haematococca, Penicillium viridicatum, Penicillium ochrochloron, or an unidentified Mucormycotina sp. increased PU degradation a further 30 to 70%, suggesting that biostimulation and bioaugmentation were operating in concert to enhance PU degradation. Interestingly, few of the inoculated fungi could be detected by DGGE in the soil or on the surface of the PU 4 weeks after inoculation. Bioaugmentation did, however, increase the numbers of indigenous PU-degrading fungi and caused an inoculum-dependent change in the composition of the native fungal populations, which may explain the increased degradation observed. These results demonstrate that both biostimulation and bioaugmentation may be viable tools for the remediation of environments contaminated with polyurethane waste.
Figures



References
-
- Alef, K., and P. Nannipieri. 1995. Enrichment, isolation and counting of soil microorganisms, p. 123-186. In K. Alef and P. Nannipieri (ed.), Methods in applied soil microbiology and biochemistry. Academic Press, London, United Kingdom.
-
- Alexander, M. 1999. Biodegradation and bioremediation. Academic Press, London, United Kingdom.
-
- Anderson, I. C., C. D. Campbell, and J. I. Prosser. 2003. Potential bias of fungal 18S rDNA and internal transcribed spacer polymerase chain reaction primers for estimating fungal biodiversity in soil. Environ. Microbiol. 5:36-47. - PubMed
-
- Baek, K. H., B. D. Yoon, B. H. Kim, D. H. Cho, I. S. Lee, H. M. Oh, and H. S. Kim. 2007. Monitoring of microbial diversity and activity during bioremediation of crude oil-contaminated soil with different treatments. J. Microbiol. Biotechnol. 17:67-73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

